| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
KSP (kinesin spindle protein) (Kiapp = 1.7 nM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
ispinesib 的 Ki app 为 1.7 nM,是一种强效且选择性极高的 KSP 抑制剂[1]。 ispinesib (150 nM) 可抑制 BT-474 和 MDA-MB-468 细胞系,GI50 分别为 45 和 19 nM[2]。 Ispinesib (SB715992, 15 和 30 nM) 分别导致前列腺癌细胞凋亡 1094.88% 和 1516.70%,并抑制 PC-3 前列腺癌细胞增殖 48.65% 和 52.16%。 ispinenesib 上调参与细胞周期停滞和凋亡的基因,而下调参与细胞存活和增殖的基因。金雀异黄素可以增强 ispinenesib 的促凋亡和抗增殖特性[3]。
Ispinesib (SB-715992)是KSP/人驱动蛋白纺锤体蛋白的强效特异性抑制剂;。 Ispinesib是KSP的变构可逆抑制剂。 Ispinesib减缓KSP与MT/微管的结合。 Ispinesib加速ATP促进的KSP与MT/微管的解离。 在MT存在下,Ispinesib的结合速度减慢。 Ispinesib在有和没有MT的情况下抑制ADP释放。 Ispinesib不会干扰mantATP与KSP-MT复合物的结合。 Ispinesib加速KSP-MT复合物中磷酸盐的释放。[1] 人类乳腺癌症细胞系体外对Ispinesib (SB-715992)的敏感性 研究人员调查了在代表不同原发性肿瘤组织类型和遗传背景的50种人类乳腺肿瘤细胞系以及三种正常乳腺上皮细胞系(MCF10A、MCF10F和MCF12A)中,特定的癌症亚型可能表现出对依匹西尼特别敏感的可能性(图1A;参考文献23)。细胞用浓度逐渐增加的ispinesib处理,并根据将生长减少50%所需的药物浓度进行排名(GI50;图1A)。所有品系的敏感性在7.4至600 nmol/L之间,其中大多数在7.4至80 nmol/L的10倍范围内。三种管腔亚型品系的灵敏度在100至600 nmool/L之间。在这个相对狭窄的敏感性范围内,我们无法发现与亚型、受体表达或突变状态有任何明显的相关性。 Ispinesib(SB-715992)抑制PC-3细胞的增殖并诱导其凋亡。发现SB715992调节与细胞增殖、细胞周期、细胞信号通路和凋亡控制相关的基因的表达。此外,我们的结果表明,与单独使用任何一种药物的效果相比,SB715992和金雀异黄素的联合治疗对细胞生长的抑制和凋亡的诱导明显更大。 |
||
| 体内研究 (In Vivo) |
在含有 ER 阳性(MCF7)、HER2 阳性(KPL4、HCC1954 和 BT-474)和三阴性(MDA-MB-468)乳腺癌细胞肿瘤异种移植物的小鼠中,Ispinesib (SB-715992)(SCID,8 mg/kg) ;裸露,10 mg/kg,q4d × 3)通过腹腔注射3次减少肿瘤体积[2]。
Ispinesib (SB-715992)作为单一药物在临床前乳腺癌症模型中的疗效[2] 为了确定在体内癌症乳腺癌模型中ispinesib抗肿瘤活性的程度,我们选择了对ispinemab表现出不同体外敏感性并代表不同亚型人类乳腺肿瘤的细胞系。它们在体外对伊西替尼的敏感性从高到低依次为:MDA-MB-468>HCC1954=MCF7>BT-474。 MCF7是一种特征明确的ER-阳性腔型癌症细胞系。MDA-MB-468是癌症基础性三阴性的模型。为了代表HER2-过表达的癌症乳腺癌,我们选择了BT-474、HCC1954和KPL4,一种转移来源的乳腺肿瘤系。这些细胞系(KPL4除外)的转录组、基因组和功能特征之前已经过表征。 携带所列系肿瘤异种移植物的小鼠按照最佳q4d×3方案,以其MTD(SCID,8mg/kg;裸,10mg/kg)腹腔注射ispinesib。Ispinesib在所有测试的模型中都很活跃(图2;表1),在每个模型中都产生了回归。然而,根据肿瘤缩小的程度、退化的数量和肿瘤再生的程度来判断,各个肿瘤的敏感性不同。 三阴性异种移植物模型MDA-MB-468是体外最敏感的品系之一(图1A),在体内表现出最大的ispinesib敏感性。在ispinesib治疗中,所有小鼠的MDA-MB-468肿瘤完全消退,在研究结束时和30天后,每只小鼠的TFS评分均为TFS(数据未显示)。 在ER阳性模型MCF7中,ispinesib在9只小鼠中的5只(1只PR和4只CR,其中2只在研究结束时为TFS)中引起了肿瘤消退,TGI为92%。 在HER2阳性模型中,KPL4对ispinesib治疗的反应最好。所有10只接受治疗的小鼠均表现出退化(4只PR、6只CR和4只TFS)。在HCC1954模型中,ispinesib导致五只接受治疗的小鼠中的四只出现退化。然而,在这两种模型中,反应较差的肿瘤在治疗后35天开始再生。在第三种HER2阳性模型BT-474中,ispinesib在8只小鼠中的2只中引起了CR,TGI(61%)低于其他模型中观察到的值,到研究结束时,所有小鼠的肿瘤都重新生长(平均肿瘤体积,875 mm3)。 MDA-MB-468异种移植物对Ispinesib (SB-715992)过敏[2] 为了进一步研究MDA-MB-468肿瘤对Ispinesib (SB-715992)的超敏反应,我们将司匹尼与ixabepilone或紫杉醇的抗肿瘤活性进行了比较,这两种抗有丝分裂疗法已被批准用于治疗癌症。我们将每种药物给药于两组携带肿瘤的动物,接受MTD或较低剂量。Ispinesib的抗肿瘤活性在TGI和回归方面与紫杉醇和伊沙匹隆相当(图3A;补充数据2)。接受高剂量依沙匹隆(5mg/kg)治疗的九只小鼠中,有一只出现肢体瘫痪,并被提前处死。紫杉醇或伊西替尼没有观察到这种毒性。 Ispinesib (SB-715992)的活性 与癌症护理标准的结合[2] 我们试图确定伊西奈西与常用于治疗癌症的药物的潜在有益组合方案:HER2靶向疗法、曲妥珠单抗和拉帕替尼、阿霉素(蒽环类)和卡培他滨(抗代谢)。在所有联合研究中,我们以MTD和最佳给药方案给药批准的药物,并根据需要调整伊西尼的剂量,以实现耐受的联合方案。 我们将ispinesib与曲妥珠单抗联合应用于两种不同的HER2过表达肿瘤模型:管腔模型BT-474(图4A)和转移衍生模型KPL4(图4B)。在这两种模型中,曲妥珠单抗没有毒性,因此可以与伊西替尼的单一药物MTD联合使用。这种组合被证明优于单一药物的治疗。在BT-474中,联合用药引起的TGI为99%,而伊西替尼和曲妥珠单抗分别为61%和88%(表2),并治愈了八只小鼠中的七只。在KPL4中,所有10只接受联合治疗的小鼠都经历了PR或CR,4只在研究结束时仍然没有肿瘤,TGI为97%。 |
||
| 酶活实验 |
人驱动蛋白纺锤体(KSP)ATP酶活性及伊匹奈西(SB-715992)抑制作用的稳态动力学分析[1]
采用丙酮酸激酶-乳酸脱氢酶偶联检测系统(通过ADP生成偶联NADH氧化)进行驱动蛋白特异性分析,监测340 nm处吸光度变化。使用基于荧光的灵敏检测系统(包含丙酮酸激酶、丙酮酸氧化酶和辣根过氧化物酶偶联体系,将ADP生成与Amplex Red氧化为荧光试卤灵相偶联)进行纳摩尔浓度KSP的稳态研究,通过荧光信号(λ激发=520 nm,λ发射=580 nm)监测试卤灵生成。稳态生化实验在PEM25缓冲液[25 mM PIPES-K+ (pH 6.8)、2 mM MgCl2、1 mM EGTA]中进行,涉及微管的实验需添加10 µM紫杉醇。稳态抑制IC50测定条件为:500 µM ATP、5 µM微管蛋白、1 nM KSP的PEM25缓冲液体系。通过浓度-响应曲线计算伊匹奈西(SB-715992)的表观抑制解离常数(Ki app),并采用Morrison方程对酶浓度进行显式校正。 伊匹奈西(SB-715992)解离半衰期(t1/2)测定[1] 通过平衡透析法测定伊匹奈西(SB-715992)从KSP解离的滞留半衰期(t1/2)。反应体系终体积1 mL(含800 nM KSP、800 nM伊匹奈西和5 µM微管蛋白),装入透析盒后于含2 mM DTT的PEM25缓冲液中透析30小时,前2小时更换三次透析液以确保充分透析。在不同时间点取样检测活性,以DMSO(无抑制剂)对照组的活性为基准进行归一化处理。通过单指数拟合实验数据获得伊匹奈西解离速率(koff),并利用公式2计算滞留半衰期(t1/2)。 瞬态动力学实验[1] 采用停流仪(SF-61 DX2)研究伊匹奈西(SB-715992)对mantATP结合、KSP与微管结合/解离、磷酸盐(Pi)释放及mantADP释放的影响。无核苷酸KSP-微管复合物的制备:将KSP与微管按1:1比例复合物与1单位/mL腺苷三磷酸双磷酸酶(apyrase)室温孵育15分钟,经20%蔗糖垫(75,000g离心15分钟)去除apyrase后,沉淀用含10 µM紫杉醇的PEM25缓冲液重悬。 每个数据点的瞬态曲线均进行三次重复采集后取平均值再拟合。mantATP/mantADP实验采用360 nm激发光与400 nm截止滤光片检测荧光发射,监测KSP结合mantATP时的荧光增强或mantADP释放导致的荧光减弱。Pi释放速率通过MDCC荧光标记的细菌磷酸盐结合蛋白(PBP)检测:将无核苷酸KSP-微管复合物、MDCC-PBP及Pi清除剂(配方见下文)与不同浓度MgATP快速混合。Pi清除剂含PNPase(10单位/mL)、7-MEG(10 mM)、MnCl2(5 µM)、葡萄糖-1,6-二磷酸(1 mM)和PDRM(1 µg/mL),其比例可消除溶液中MDCC-PBP对Pi的竞争。通过340 nm处浊度变化监测KSP-微管复合物的解离/结合动力学。KSP从微管解离速率实验如前述方法采用MgATP触发:预先形成无核苷酸KSP-微管复合物(存在/不存在伊匹奈西(SB-715992)),在停流仪中与含不同浓度MgATP(0–500 µM)及100 mM KCl的PEM25缓冲液快速混合。该设计中KSP通过ATP结合水解从微管脱离,额外添加的KCl可减弱马达蛋白与微管的相互作用以减少再结合。 通过监测Trp127荧光测定伊匹奈西与KSP的结合速率。在不同生理状态(KSP-微管、KSP-AMPPMP-微管(ATP类似态)、KSP-ADP和KSP-AMPPNP)下,通过loop5区域Trp127荧光淬灭监测伊匹奈西结合速率(激发波长295 nm,发射滤光片截止320 nm)。将伊匹奈西母液(0–100 µM)与含1 µM KSP(或KSP-微管复合物)及500 µM ADP/AMP-PNP的PEM25缓冲液通过停流仪混合,荧光淬灭曲线采用双指数方程拟合。 |
||
| 细胞实验 |
蛋白质印迹分析(Western blot)[2]
用150 nmol/L 伊匹奈西(SB-715992)处理细胞后,使用RIPA裂解液提取蛋白。采用Bax、Bid、xIAP、Bcl2、磷酸化Bcl2(Ser70)及Bcl-XL(54H6)一抗,其他一抗包括周期蛋白B(cyclin B)和周期蛋白E(cyclin E,HE12)。二抗为IR 680/800CW LI-COR,信号检测与分析通过LI-COR Odyssey成像系统完成。 流式细胞术DNA细胞周期分析[2] 用150 nmol/L 伊匹奈西(SB-715992)处理细胞后,以85%冰乙醇固定,重悬于含10 μg/mL碘化丙啶(PI)DNA染料及250 μg/mL RNase A的PBS中,通过FACSCalibur流式细胞仪检测,数据采用FlowJo软件分析。 细胞增殖抑制实验[3] 将PC-3前列腺癌细胞以4×10³个/孔密度接种于96孔板,孵育24小时使贴壁。实验分为三组:1)分别用15 nM和30 nM 伊匹奈西(SB-715992)处理;2)联合处理组(7.5或10 nM SB715992 + 30 μM染料木黄酮);3)预加30 μM染料木黄酮24小时后,再给予15 nM SB715992。对照组使用0.3 mM Na₂CO₃(溶剂对照)。处理后,细胞与MTT(0.5 mg/mL)37℃孵育2小时,异丙醇室温处理1小时。 DNA ladder法检测细胞凋亡[3] 将PC-3细胞(3.5×10⁵个/皿)接种于100 mm培养皿,培养36小时后,用15 nM 伊匹奈西(SB-715992)处理48及72小时。裂解细胞(10 mM Tris pH 8.0、0.5 mM EDTA、0.2% Triton X-100),13,800×g 4℃离心15分钟分离胞质DNA片段。上清经RNase(15 μL)37℃消化1小时,再加入20% SDS(20 μL)、蛋白酶K(8 μL,20 mg/mL)、5.0 M NaCl(25 μL)37℃孵育30分钟。酚/氯仿/异戊醇抽提后异丙醇沉淀DNA,70%乙醇洗涤,1.5%琼脂糖凝胶(100 V,80分钟)电泳,溴化乙锭染色后紫外成像。 基因表达谱芯片分析[3] 用10 nM 伊匹奈西(SB-715992)处理PC-3细胞6、24、48小时后,Trizol法提取总RNA,RNeasy Mini Kit纯化后,通过Human Genome U133A芯片(含54,613个人类基因探针)进行检测。使用Microarray Suite、MicroDB™及Data Mining Tool软件量化表达,Cluster和TreeView进行聚类分析,Onto-Express与GenMAPP完成功能注释。 逆转录聚合酶链反应(RT-PCR)验证RNA表达[3] 为验证芯片结果,选取差异表达基因(表1和表2)进行实时定量PCR。10 nM 伊匹奈西(SB-715992)处理PC-3细胞6及48小时后,提取总RNA并反转录(Superscript cDNA合成试剂盒)。25 μL反应体系(2 μL cDNA、12.5 μL 2× SYBR Green Mix、1.5 μL 5 μM正/反向引物、7.5 μL H₂O)于SmartCycler II中扩增(95℃ 10分钟;95℃ 15秒→60℃ 1分钟,40循环)。采用ΔCt法分析数据,以β-肌动蛋白(actin)为内参,熔解曲线确认扩增特异性。 蛋白质印迹分析[3] PC-3细胞(3.0×10⁵个/皿)接种100 mm培养皿24小时后,用10 nM 伊匹奈西(SB-715992)处理24及48小时。裂解细胞(62.5 mM Tris-HCl、2% SDS),BCA法测定蛋白浓度。取等量蛋白经10%或14% SDS-PAGE分离,100 V 4℃转膜2小时。膜依次与抗EGFR、抗p27(1:100)、抗p15(1:200)及抗β-actin(1:10,000)一抗孵育,再与辣根过氧化物酶偶联二抗反应。 |
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Descriptive PK parameters were calculated using noncompartmental methods. Because of a sparse sampling scheme, the full PKs of ispinesib cannot be completely described. Available PK data (Table 4) suggest that systemic exposure of ispinesib was not dose proportional over the dose range of 10–14 mg/m2. The mean Cmax (C1 h/end of infusion) and AUClast on day 1 were 349±107 ng/ml (10 mg/m2), 223±48.5 ng/ml (12 mg/m2), and 213±109 ng/ml (14 mg/m2). On day 15, the mean Cmax and AUClast were 396±129 ng/ml (10 mg/m2), 248±136 ng/ml (12 mg/m2), and 230±68.7 ng/ml (14mg/m2). There was no significant difference in drug exposure between days 1 and 15. https://pubmed.ncbi.nlm.nih.gov/22123335/
|
||
| 毒性/毒理 (Toxicokinetics/TK) |
Determination of maximum tolerated dose and safety results:
Two DLTs, both transient grade 3 aspartate aminotransferase (AST) and ALT increases, were observed at 14 mg/m2. Both patients remained in the study without dose reduction without further toxicity. These were the only two DLTs reported in the study. The MTD was determined to be 12 mg/m2. Table 3 summarizes AEs reported in one or more patients during the study. The most common AEs, reported in at least three patients, included neutropenia (n=14, 87.5%), increased ALT (n=9, 56.3%), anemia (n=6, 37.5%), increased AST and diarrhea (n=5, 31.3%), increased alkaline phosphatase (n=4, 25.0%), and leukopenia, thrombocythemia, nausea, vomiting, headache, and breast pain (n=3, 18.8%). No AEs of neuropathy, mucositis, or alopecia were reported. The only grade 3 and grade 4 AEs reported were neutropenia (grade 3, n=6, 37.5%; grade 4, n=7, 43.8%), ALT and AST increased (grade 3, n=2, 12.5%), and febrile neutropenia (grade 3), thrombocytopenia (grade 4; likely a laboratory error), pleural effusion (grade 3), and spontaneous abortion (grade 4), each n=1, 6.3%. The spontaneous abortion occurred in a patient who had a negative pregnancy test at study entry but was found to be pregnant following the completion of cycle 1 dosing. Because of the pregnancy, post-treatment imaging was not performed and the patient was excluded from the study. There were no grade 5 AEs. No notable changes in ECG measurements, including Bazett’s corrected Q–T interval, vital signs, or physical exam including neurological assessments, were observed. https://pubmed.ncbi.nlm.nih.gov/22123335/ |
||
| 参考文献 | |||
| 其他信息 |
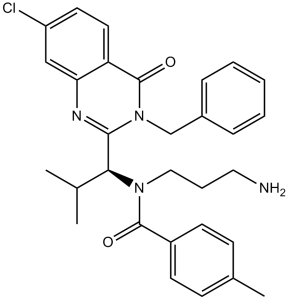
N-(3-aminopropyl)-N-[(1R)-1-[7-chloro-4-oxo-3-(phenylmethyl)-2-quinazolinyl]-2-methylpropyl]-4-methylbenzamide is a member of benzamides.
Ispinesib is a synthetic small molecule, derived from quinazolinone, with antineoplastic properties. Ispinesib selectively inhibits the mitotic motor protein, kinesin spindle protein (KSP), resulting in inhibition of mitotic spindle assembly, induction of cell cycle arrest during the mitotic phase, and cell death in tumor cells that are actively dividing. Because KSP is not involved in nonmitotic processes, such as neuronal transport, ispinesib may be less likely to cause the peripheral neuropathy often associated with the tubulin-targeting agents. Drug Indication Investigated for use/treatment in breast cancer, lung cancer, solid tumors, renal cell carcinoma, pediatric indications, ovarian cancer, and head and neck cancer. KSP, also known as HsEg5, is a kinesin that plays an essential role in the formation of a bipolar mitotic spindle and is required for cell cycle progression through mitosis. Ispinesib is the first potent, highly specific small-molecule inhibitor of KSP tested for the treatment of human disease. This novel anticancer agent causes mitotic arrest and growth inhibition in several human tumor cell lines and is currently being tested in multiple phase II clinical trials. In this study we have used steady-state and pre-steady-state kinetic assays to define the mechanism of KSP inhibition by ispinesib. Our data show that ispinesib alters the ability of KSP to bind to microtubules and inhibits its movement by preventing the release of ADP without preventing the release of the KSP-ADP complex from the microtubule. This type of inhibition is consistent with the physiological effect of ispinesib on cells, which is to prevent KSP-driven mitotic spindle pole separation. A comparison of ispinesib to monastrol, another small-molecule inhibitor of KSP, reveals that both inhibitors share a common mode of inhibition. [1] Purpose: Ispinesib (SB-715992) is a potent inhibitor of kinesin spindle protein, a kinesin motor protein essential for the formation of a bipolar mitotic spindle and cell cycle progression through mitosis. Clinical studies of ispinesib have shown a 9% response rate in patients with locally advanced or metastatic breast cancer and a favorable safety profile without significant neurotoxicities, gastrointestinal toxicities, or hair loss. To better understand the potential of ispinesib in the treatment of breast cancer, we explored the activity of ispinesib alone and in combination with several therapies approved for the treatment of breast cancer. Experimental design: We measured the ispinesib sensitivity and pharmacodynamic response of breast cancer cell lines representative of various subtypes in vitro and as xenografts in vivo and tested the ability of ispinesib to enhance the antitumor activity of approved therapies. Results: In vitro, ispinesib displayed broad antiproliferative activity against a panel of 53 breast cell lines. In vivo, ispinesib produced regressions in each of five breast cancer models and tumor-free survivors in three of these models. The effects of ispinesib treatment on pharmacodynamic markers of mitosis and apoptosis were examined in vitro and in vivo, revealing a greater increase in both mitotic and apoptotic markers in the MDA-MB-468 model than in the less sensitive BT-474 model. In vivo, ispinesib enhanced the antitumor activity of trastuzumab, lapatinib, doxorubicin, and capecitabine and exhibited activity comparable with paclitaxel and ixabepilone. Conclusions: These findings support further clinical exploration of kinesin spindle protein inhibitors for the treatment of breast cancer. [2] Background: Kinesin spindle proteins (KSP) are motor proteins that play an essential role in mitotic spindle formation. HsEg5, a KSP, is responsible for the formation of the bipolar spindle, which is critical for proper cell division during mitosis. The function of HsEg5 provides a novel target for the manipulation of the cell cycle and the induction of apoptosis. SB715992, an experimental KSP inhibitor, has been shown to perturb bipolar spindle formation, thus making it an excellent candidate for anti-cancer agent. Our major objective was a) to investigate the cell growth inhibitory effects of SB715992 on PC-3 human prostate cancer cell line, b) to investigate whether the growth inhibitory effects of SB715992 could be enhanced when combined with genistein, a naturally occurring isoflavone and, c) to determine gene expression profile to establish molecular mechanism of action of SB715992. Methods: PC-3 cells were treated with varying concentration of SB715992, 30 microM of genistein, and SB715992 plus 30 microM of genistein. After treatments, PC-3 cells were assayed for cell proliferation, induction of apoptosis, and alteration in gene and protein expression using cell inhibition assay, apoptosis assay, microarray analysis, real-time RT-PCR, and Western Blot analysis. Results: SB715992 inhibited cell proliferation and induced apoptosis in PC-3 cells. SB715992 was found to regulate the expression of genes related to the control of cell proliferation, cell cycle, cell signaling pathways, and apoptosis. In addition, our results showed that combination treatment with SB715992 and genistein caused significantly greater cell growth inhibition and induction of apoptosis compared to the effects of either agent alone. Conclusion: Our results clearly show that SB715992 is a potent anti-tumor agent whose therapeutic effects could be enhanced by genistein. Hence, we believe that SB715992 could be a novel agent for the treatment of prostate cancer with greater success when combined with a non-toxic natural agent like genistein. [3] The objective of the study was to evaluate the safety, pharmacokinetics, and antitumor activity of ispinesib, a kinesin spindle protein inhibitor. Patients with locally advanced or metastatic breast cancer who had received only prior neoadjuvant or adjuvant chemotherapy were treated with escalating doses of ispinesib administered as a 1-h infusion on days 1 and 15 every 28 days until toxicity or progression of disease. Doses were escalated until dose-limiting toxicity was observed in two out of six patients during cycle 1. A total of 16 patients were treated at three dose levels: 10 mg/m (n=3), 12 mg/m (n=6), and 14 mg/m (n=7). Forty-four percent of the patients had locally advanced disease and 56% had metastatic disease; 50% were estrogen receptor positive, 44% were progesterone receptor positive, 25% human epidermal growth factor 2 were positive, and 31% triple (estrogen receptor, progesterone receptor, human epidermal growth factor 2) negative. Sixty-nine percent of patients were chemo-naive. The maximum tolerated dose was 12 mg/m and dose-limiting toxicity was grade 3 increased aspartate aminotransferase and alanine aminotransferase. The most common toxicities included neutropenia (88%; 38% grade 3 and 44% grade 4), increased alanine aminotransferase (56%), anemia (38%), increased aspartate aminotransferase (31%), and diarrhea (31%). No neuropathy, mucositis, or alopecia was reported. Among the 15 patients evaluable for antitumor activity, there were three partial responses, one confirmed by the response evaluation criteria in solid tumors (7% response rate). Nine patients (60%) had stable disease lasting at least 42 days, with four (27%) lasting for at least 90 days. Disease stabilization (partial responses+stable disease) was observed in 11 (73.3%) patients. In conclusion, ispinesib was well tolerated when administered on days 1 and 15 every 28 days. Limited activity was observed with this schedule in patients with previously untreated advanced breast cancer. Anticancer Drugs . 2012 Mar;23(3):335-41. |
| 分子式 |
C30H33CLN4O2
|
|
|---|---|---|
| 分子量 |
517.06
|
|
| 精确质量 |
516.229
|
|
| 元素分析 |
C, 69.69; H, 6.43; Cl, 6.86; N, 10.84; O, 6.19
|
|
| CAS号 |
336113-53-2
|
|
| 相关CAS号 |
336113-53-2;514820-03-2 (mesylate);
|
|
| PubChem CID |
6851740
|
|
| 外观&性状 |
White to light yellow solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
708.0±70.0 °C at 760 mmHg
|
|
| 闪点 |
382.0±35.7 °C
|
|
| 蒸汽压 |
0.0±2.3 mmHg at 25°C
|
|
| 折射率 |
1.619
|
|
| LogP |
5.2
|
|
| tPSA |
81.22
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
9
|
|
| 重原子数目 |
37
|
|
| 分子复杂度/Complexity |
803
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
CC1=CC=C(C=C1)C(=O)N(CCCN)[C@@H](C2=NC3=C(C=CC(=C3)Cl)C(=O)N2CC4=CC=CC=C4)C(C)C
|
|
| InChi Key |
QJZRFPJCWMNVAV-HHHXNRCGSA-N
|
|
| InChi Code |
InChI=1S/C30H33ClN4O2/c1-20(2)27(34(17-7-16-32)29(36)23-12-10-21(3)11-13-23)28-33-26-18-24(31)14-15-25(26)30(37)35(28)19-22-8-5-4-6-9-22/h4-6,8-15,18,20,27H,7,16-17,19,32H2,1-3H3/t27-/m1/s1
|
|
| 化学名 |
(R)-N-(3-aminopropyl)-N-(1-(3-benzyl-7-chloro-4-oxo-3,4-dihydroquinazolin-2-yl)-2-methylpropyl)-4-methylbenzamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.84 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.84 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9340 mL | 9.6701 mL | 19.3401 mL | |
| 5 mM | 0.3868 mL | 1.9340 mL | 3.8680 mL | |
| 10 mM | 0.1934 mL | 0.9670 mL | 1.9340 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。