| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
0.3 至 3 μM 的兰索拉唑以浓度依赖性方式抑制胃酸的产生(IC50 为 0.76 μM)[4]。兰索拉唑 (30–300 μM) 可诱导浓度依赖性、可逆且可重复的动脉舒张 [5]。
|
|---|---|
| 体内研究 (In Vivo) |
兰索拉唑(20-40 mg/kg)治疗可显着改善记忆障碍以及 STZ 和 HFD 引起的生化和组织学改变 [3]。兰索拉唑的口服剂量(20 mg/kg 和 40 mg/kg)可显着降低 STZ 和 HFD 引起的 AChE 活性升高 [3]。兰索拉唑口服剂量(20 mg/kg 和 40 mg/kg)可显着降低 STZ 和 HFD 引起的脑 MPO 水平升高 [3]。与对照动物相比,其他口服兰索拉唑(20 mg/kg 和 40 mg/kg)的 HFD 小鼠体重减轻了很多 [3]。
|
| 动物实验 |
Animal/Disease Models: Swiss albino mice (20–25 g) of either sex[3].
Doses: 20 mg/kg, 40 mg/kg. Route of Administration: PO, started after second dose of STZ and then subjected to MWM test. Continued ( 30 min before) during the acquisition trial conducted from day 1 to day 4. Experimental Results: Dramatically attenuated the day 4 rise in ELT and diminished in day 5 TSTQ in the STZ as well as HFD treated mice in a dose dependent manner. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The oral bioavailability of lansoprazole is reported to be 80-90% and the peak plasma concentration(Cmax) is achieved about 1.7 hours after oral dosing. Food reduces the absorption of lansoprazole (both Cmax and AUC are reduced by 50-70%); therefore, patients should be instructed to take lansoprazole before meals. A reported 14-23% of a lansoprazole is eliminated in the urine with this percentage range including both conjugated and unconjugated hydroxylated metabolites. The apparent volume of distribution of lansoprazole is 0.4 L/kg. The reported clearance of lansoprazole is 400-650 mL/min. Very high (around 97%) /protein binding/; protein binding remains constant over the concentration range of 0.05 to 5 ug/mL. In patient with renal function impairment, protein binding may be decreased by 1 to 1.5%. Distributed in tissue, particularly gastric parietal cells. Apparent oral volume of distribution following administration of 30 mg of lansoprazole is about 0.5 L/kg. Since lansoprazole is acid-labile, it is administered as a capsule containing enter-coated granules to prevent gastric decomposition and to increase bioavailability. Once lansoprazole has left the stomach, absorption is rapid and relatively complete, with absolute bioavailability over 80%. Bioavailability may be decreased if lansoprazole is administered within 30 minutes of food intake as compared to that of a fasting state. Absorption may be delayed in patients with hepatic cirrhosis. Elimination: Renal: Approximately 14 to 25% of a dose of lansoprazole is excreted in the urine, as conjugated and unconjugated hydroxylated metabolites. Less than 1% of unchanged lansoprazole is detectable in the urine. Biliary/fecal: Approximately two-thirds of a dose of lansoprazole is detected as metabolites in the feces. In dialysis: Lansoprazole and its metabolites are not significantly dialyzed; no appreciable fraction is removed by hemodialysis. Note: Elimination is prolonged in healthy elderly subjects, in adult and elderly patient with mild renal impairment, and in patients with severe liver disease. For more Absorption, Distribution and Excretion (Complete) data for LANSOPRAZOLE (6 total), please visit the HSDB record page. Metabolism / Metabolites Lansoprazole is predominantly metabolized in the liver by CYP3A4 and CYP2C19. The resulting major metabolites are 5-hydroxy lansoprazole and the sulfone derivative of lansoprazole. Lansoprazole is extensively metabolized in the liver to two main excretory metabolites that are inactive. In the acidic environment of the gastric parietal cell, lansoprazole is converted to two active compounds that inhibit acid secretion by (H+,K+)-ATPase within the parietal cell canaliculus, but that are not present in the systemic circulation. Biological Half-Life One source reports the half life of lansoprazole to be 0.9 - 1.6 hours, while another source cites 0.9 - 2.1 hours. The general consensus is that lansoprazole has a short half life and is approximately 2 hours or less. These numbers may be misleading since it suggests that lansoprazole has a short duration of action when in practice, lansoprazole can effectively inhibit acid secretion for ~24 hours due to it's mechanism of action. Elimination: Normal renal function: Approximately 1.5 hours. Renal function impairment: Shortened elimination half-life. Elderly patients: 1.9 to 2.9 hours. Hepatic function impairment: 3.2 to 7.2 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Despite its wide use, lansoprazole has only rarely been associated with hepatic injury. In large scale, long term trials of lansoprazole, serum ALT elevations have occurred in less than 1% of patients and at rates similar to those that occur with placebo or comparator drugs. Only a small number of cases of clinically apparent liver disease due to lansoprazole or dexlansoprazole have been published and most have been anicteric and mild. In most instances, the time to onset was within 2 to 4 weeks and the pattern of enzyme elevations was hepatocellular or mixed. Hypersensitivity reactions with fever, rash and eosinophilia have been described due to dexlansoprazole and lansoprazole, and these reactions may be accompanied by minor serum enzyme elevations and thus qualify for DRESS syndrome (drug-rash with eosinophilia and systemic symptoms). Autoantibody formation is rare. Recovery is usually rapid (within a month) and complete upon stopping lansoprazole. Recurrence on reexposures has been reported. Likelihood score: C (probable rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of lansoprazole during breastfeeding. However, lansoprazole has been used safely in newborn infants, so it is unlikely that the amount in breastmilk would be harmful. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk The Spanish pharmacovigilance system found 3 cases of gynecomastia associated with lansoprazole reported during the time period of 1982 to 2006. A retrospective claims database study in the United States found that users of proton pump inhibitors had an increased risk of gynecomastia. A review article reported that a search of database from the European Pharmacovigilance Centre found 45 cases of gynecomastia, 11 cases of galactorrhea, 3 cases of breast pain and 5 cases of breast enlargement associated with lansoprazole. A search of the WHO global pharmacovigilance database found 123 cases of gynecomastia, 30 cases of galactorrhea, 36 cases of breast pain and 18 cases of breast enlargement associated with lansoprazole. In a retrospective study, out of total of 127 cases of gynecomastia in of 175 male patients, 11 patients were treated had been treated with lansoprazole. One case of elevated serum prolactin and galactorrhea was reported in a 21-year-old man. When omeprazole was substituted for lansoprazole, the serum prolactin decreased to the normal range and galactorrhea ceased. Although this case occurred in Spain, it was not included in the report above. A 13-year-old girl with a recent history of bilateral galactorrhea and hyperprolactinemia from omeprazole and domperidone on separate occasions was given lansoprazole to prevent gastrointestinal irritation following intravenous diclofenac for a severe headache. After 3 days of lansoprazole therapy, she again developed galactorrhea and an elevated serum prolactin that returned to normal a week after discontinuing lansoprazole. A 17-year-old woman using a progestin-containing IUD for 1 year began lansoprazole 15 mg daily and presented after 1 week with bilateral galactorrhea and hyperprolactinemia of 92 mcg/L. Seventy-two hours after discontinuation of lansoprazole, galactorrhea ceased. Four months later, serum prolactin was normal at 24.1 mcg/L with no recurrence of galactorrhea. The authors judged the adverse reaction likely to be caused by lansoprazole. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding 97% of lansoprazole is plasma protein bound. Interactions Possible interactions of lansoprazole with medications known to be metabolized by the hepatic cytochrome p450 enzyme system should be considered. ... Lansoprazole, by increasing gastric pH, as the potential to affect the bioavailability of any medication whose absorption is pH-dependent. Also, lansoprazole may prevent the degradation of acid-labile drugs. Lansoprazole causes prolonged inhibition of gastric acid secretion, and thereby my interfere with the absorption of these medications /ampicillin ester, digoxin, iron salts, ketoconazole/and other for which bioavailability is determined by gastric pH. Lansoprazole appears to produce a dose-dependent decrease in the absorption of cyanocobalamin; this my be due to lansoprazole-induced hypochlorhydria or achlorhydria. For more Interactions (Complete) data for LANSOPRAZOLE (9 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Antiulcerative Lansoprazole is indicated for the short-term treatment of heartburn and other symptoms associated with gastroesophageal reflux disease (GERD). Lansoprazole is indicated for the short-term (up to 8 weeks) treatment for symptom relief and healing of all grades of erosive esophagitis (associated with GERD). Lansoprazole may be indicated for an additional 8 weeks of treatment of patients in whom healing has not occurred. If erosive esophagitis recurs, an additional course of lansoprazole treatment may be considered. Lansoprazole also is indicated to maintain healing of erosive esophagitis. /Included in US product labeling/ Lansoprazole is indicated for short-term (up to 8 weeks) treatment in patients with active benign gastric ulcer. /Included in US product labeling/ Lansoprazole is indicated for short-term (up to 4 weeks) treatment for symptom relief and healing in patients with active duodenal ulcer. Lansoprazole also is indicated to maintain healing of duodenal ulcers. /Included in US product labeling/ For more Therapeutic Uses (Complete) data for LANSOPRAZOLE (6 total), please visit the HSDB record page. Drug Warnings Worldwide, over 10,000 patients have been treated with lansoprazole in Phase 2-3 trials involving various dosages and durations of treatment. The adverse reaction profiles for prevacid delayed-release capsules and prevacid for delayed-release oral suspension are similar. In general, lansoprazole treatment has been well-tolerated in both short-term and long-term trials. ... The most commonly reported possibly or probably treatment-related adverse event during maintenance therapy was diarrhea. An 85-year-old white man presented with an upper gastrointestinal hemorrhage from a gastric ulcer. His platelet count was normal on admission. He was started on oral lansoprazole 60 mg twice daily and, on hospital day 2, his platelet count decreased to 102 x 10(3)/mm(3); on hospital day 3, the platelet count was 36 x 10(3)/mm(3). Lansoprazole was discontinued, and the platelet count returned to normal. He has not had any further episodes of thrombocytopenia to date. After exclusion of other causes, the onset of thrombocytopenia after administration of lansoprazole, the resolution of the adverse reaction after discontinuation of the drug, and the fact that no other medicines were introduced during this time frame lead us to believe that this was most likely an idiosyncratic thrombocytopenic response to lansoprazole. To date, this is the first reported case of what appears to be isolated thrombocytopenia associated with lansoprazole. Studies in elderly patients indicate that the clearance of lansoprazole is decreased in the elderly, resulting in a 50 to 100% increase in the elimination half-life. Because the mean half-life in the elderly remains between 1.9 and 2.9 hours, repeated once-daily dosing does not result in accumulation of lansoprazole. However, subsequent doses higher than 30 mg a day should not be administered unless additional gastric acid suppression is necessary. Diarrhea is one of the most frequently reported adverse events during proton pump inhibitor use in any setting. Because of the limited available information, this study was set up with the aim of assessing the incidence and characteristics of diarrhea and to investigate possible associated co-factors in proton pump inhibitor users in daily practice. Data were used from a prospective, observational study in which 10,008 lansprazole users were followed over time (1994-1998). The study was designed according to the SAMM guidelines. A nested case-control design was used to compare proton pump inhibitor users reporting diarrhea with those reporting no diarrhea. The frequency of diarrhea was 3.7% and the incidence density 10.7 per 1000 patients months of proton pump inhibitor use. The diarrhea was most commonly loose and occurred on average 4.4 times per day. The analysis of co-factors revealed that patients with concomitant use of oral antibiotics and patients reporting neurological and/or dermatological adverse events, were at risk of developing diarrhea during proton pomp inhibitor use. For more Drug Warnings (Complete) data for LANSOPRAZOLE (8 total), please visit the HSDB record page. Pharmacodynamics Lansoprazole decreases gastric acid secretion by targeting H+,K+-ATPase, which is the enzyme that catalyzes the final step in the acid secretion pathway in parietal cells. Conveniently, lansoprazole administered any time of day is able to inhibit both daytime and nocturnal acid secretion. The result is that lansoprazole is effective at healing duodenal ulcers, reduces ulcer-related pain, and offers relief from symptoms of heartburn Lansoprazole also reduces pepsin secretion, making it a useful treatment option for hypersecretory conditions such as Zollinger-Ellison syndrome. |
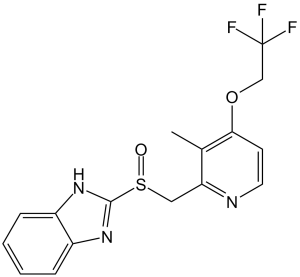
| 分子式 |
C16H14F3N3O2S
|
|
|---|---|---|
| 分子量 |
369.36
|
|
| 精确质量 |
369.075
|
|
| CAS号 |
103577-45-3
|
|
| 相关CAS号 |
(R)-Lansoprazole;138530-94-6;Lansoprazole-d4;934294-22-1;(S)-Lansoprazole;138530-95-7
|
|
| PubChem CID |
3883
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 沸点 |
555.8±60.0 °C at 760 mmHg
|
|
| 熔点 |
178-182°C dec.
|
|
| 闪点 |
289.9±32.9 °C
|
|
| 蒸汽压 |
0.0±1.5 mmHg at 25°C
|
|
| 折射率 |
1.635
|
|
| LogP |
2.76
|
|
| tPSA |
87.08
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
8
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
25
|
|
| 分子复杂度/Complexity |
480
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
MJIHNNLFOKEZEW-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C16H14F3N3O2S/c1-10-13(20-7-6-14(10)24-9-16(17,18)19)8-25(23)15-21-11-4-2-3-5-12(11)22-15/h2-7H,8-9H2,1H3,(H,21,22)
|
|
| 化学名 |
2-[[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl]-1H-benzimidazole
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.77 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.77 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7074 mL | 13.5369 mL | 27.0739 mL | |
| 5 mM | 0.5415 mL | 2.7074 mL | 5.4148 mL | |
| 10 mM | 0.2707 mL | 1.3537 mL | 2.7074 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Study to Evaluate the Efficacy and Safety of Ilaprazole 10 mg in Prevention NSAIDs Associated Peptic Ulcer
CTID: NCT06284876
Phase: Phase 3 Status: Not yet recruiting
Date: 2024-02-29
|
|---|
|