| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|

| 靶点 |
ErbB2 (IC50 = 9.2 nM); EGFR (IC50 = 10.8 nM); ErbB4 (IC50 = 367 nM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:Lapatinib Ditosylate 弱抑制 ErbB4 的活性,IC50 为 367 nM,并且对 EGFR 和 ErbB2 的选择性比其他激酶(例如 c-Src、c-Raf、MEK、ERK、c-Fms、 CDK1、CDK2、p38、Tie-2 和 VEGFR2。 Lapatinib Ditosylate 以剂量依赖性方式显着抑制 EGFR 和 ErbB2 受体自身磷酸化,在 HN5 细胞中 IC50 分别为 170 nM 和 80 nM;在 BT474 细胞中分别为 210 nM 和 60 nM。与优先抑制 EGFR 过表达细胞生长的 OSI-774 和 Iressa (ZD1839) 不同,二甲苯磺酸拉帕替尼同时抑制 EGFR 和 ErbB2 过表达细胞的生长。与表达低水平 EGFR 或 ErbB2 的细胞(IC50 为 3-12 μM)相比,二甲苯磺酸拉帕替尼对 EGFR 或 ErbB2 过表达细胞表现出更高的抑制活性,IC50 为 0.09-0.21 μM,并且选择性比正常细胞高约 100 倍成纤维细胞。 Lapatinib Ditosylate 有效抑制 EGFR 过表达的 HN5 和 A-431 细胞以及 ErbB2 过表达的 BT474 和 N87 细胞的生长,并显着诱导 HN5 细胞的 G1 停滞和 BT474 细胞的凋亡,这与抑制 AKT 磷酸化有关。激酶测定:通过测量肽底物磷酸化的抑制来产生酶活性抑制的IC50值。 EGFR 和 ErbB2 的胞内激酶结构域从杆状病毒表达系统中纯化。 EGFR 和 ErbB2 反应在 96 孔聚苯乙烯圆底板中进行,最终体积为 45 μL。反应混合物含有 50 mM 4-吗啉丙磺酸 (pH 7.5)、2 mM MnCl2、10 μM ATP、1 μCi 的 [γ33P] ATP/反应、50 μM 肽 A [生物素-(氨基己酸)-EEEEYFELVAKKK-CONH2]、 1 mM 二硫苏糖醇和 1 μL DMSO,其中含有从 10 μM 开始的拉帕替尼连续稀释液。通过添加指定的纯化 1 型受体胞内结构域来启动反应。添加的酶量为 1 pmol/反应 (20 nM)。 23°C 10 分钟后,加入 45 μL 0.5% 磷酸水溶液终止反应。将终止的反应混合物(75 μL)转移至磷酸纤维素过滤板。将板过滤并用 200 μL 0.5% 磷酸洗涤 3 次。将闪烁混合物 (50 μL) 添加到每个孔中,并通过在 Packard Topcount 中计数来量化测定。 IC50 值由 10 点剂量反应曲线生成。细胞测定:将细胞暴露于不同浓度的拉帕替尼72小时。使用亚甲蓝染色估计相对细胞数。在 Spectra 酶标仪中读取 620 nm 处的吸光度。通过碘化丙啶染色和掺入的 BrdUrd 的抗体检测以及碘化丙啶染色来评估细胞死亡和细胞周期分析。
|
| 体内研究 (In Vivo) |
每天两次口服二甲苯磺酸拉帕替尼(~100 mg/kg)可显着抑制 BT474 和 HN5 异种移植物的生长,且呈剂量依赖性。
拉帕替尼联合放射治疗可抑制小鼠MBT-2异种移植瘤的生长[2] 在移植肿瘤的小鼠中,连续7天每日剂量的拉帕替尼(200 mg/kg/天)与第4天的放疗联合抑制肿瘤生长的程度比单独放疗更大。 在该动物模型中,拉帕替尼治疗异种移植物肿瘤的结果显示,与单独照射相比,拉帕替尼每日剂量(口服,200 mg/kg/天)连续7天,第4天联合放疗可显著抑制异种移植物肿瘤的生长(图6A)。然而,单独口服拉帕替尼治疗效果极小。结果表明,口服拉帕替尼可使放射介导的对异种移植肿瘤的抑制增加约60%。7天治疗方案结束后,免疫组化检测小鼠肿瘤中HER-2和EGFR的表达结果显示,放疗参与了EGFR和HER-2水平的提高(图6B)。然而,拉帕替尼联合放射治疗抑制了外种移植物肿瘤中放射介导的EGFR和HER-2的激活。该体内实验结果表明,拉帕替尼除了促进DNA损伤外,还通过抑制辐射介导的EGFR和HER-2的表达来诱导放射致敏。[2] |
| 酶活实验 |
测量肽底物磷酸化抑制的过程产生酶活性抑制的 IC50 值。使用杆状病毒表达系统分离 EGFR 和 ErbB2 胞内激酶结构域。在圆底聚苯乙烯 96 孔板中,以 45 μL 的终体积进行 EGFR 和 ErbB2 反应。反应混合物由以下成分组成:50 μM 肽 A [生物素-(氨基己酸)-EEEEYFELVAKKK-CONH2]、1 mM 二硫苏糖醇、2 mM MnCl2、10 μM ATP、1 μCi [γ33P] ATP/反应,以及 1 μL DMSO,其中含有从 10 μM 开始的拉帕替尼系列稀释液。添加指定的纯化 1 型受体胞内结构域以开始反应。每个反应使用 1 pmol 添加的酶 (20 nM)。 23°C 10 分钟后,加入 45 μL 0.5% 磷酸水溶液以终止反应。将 75 μL 成品反应混合物置于磷酸纤维素滤板上。将板进行三轮过滤并用 200 μL 0.5% 磷酸洗涤。每个孔接收 50 μL 闪烁混合物,并使用 Packard Topcount 进行测定。 10 点剂量反应曲线用于计算 IC50 值。
|
| 细胞实验 |
拉帕替尼以不同浓度作用于细胞,持续时间为 72 小时。用亚甲基蓝染色可估计相对细胞数。使用 Spectra 酶标仪测量 620 nm 处的吸光度。通过使用碘化丙啶染色、掺入的 BrdUrd 的抗体检测以及碘化丙啶染色、细胞死亡和细胞周期分析进行评估。
克隆生成试验(菌落形成试验)[2] 为了测试lapatinib /拉帕替尼和辐照对集落形成的影响,细胞接种于六孔板,细胞密度为1×105 cells/well。细胞暴露于不同剂量的辐射下,但用拉帕替尼(200-1,000 nM)预处理30 min,对照细胞用二甲基亚砜(DMSO)处理。经拉帕替尼预处理和辐照后,细胞再培养一周。使用光学显微镜(×100放大)进行细胞菌落计数,菌落定义为50个或更多细胞的一组。 细胞周期分析[2] 流式细胞术分析细胞周期分布。分析细胞DNA的碘化丙啶(PI)染色。在该方案中,106个细胞/ml暴露于拉帕替尼和如上所述的辐照下,离心后收集。用PI (15 μg/ml)、5 μg/ml无dna核糖核酸酶(DNase-free RNase)和Tween-20(0.5%)在PBS中染色。使用tune™NxT声学聚焦细胞仪对样品进行分析。 免疫荧光显微镜研究[2] 将MBT-2细胞转移到预先涂有聚赖氨酸的盖子上12小时,使细胞附着在表面。细胞单独暴露于2.5 Gy的辐射剂量,或与100 nM的lapatinib 联合暴露。细胞孵育45分钟,用冷冻PBS洗涤3次,然后用4%的甲醛PBS溶液固定30分钟,然后用0.5% Triton X-100/PBS孵育60分钟,5%牛血清白蛋白(BSA)孵育60分钟,最后用异硫氰酸荧光素(FITC)偶联抗磷酸化组蛋白γ-H2AX抗体(1:15 00)孵育2小时。用PBS洗涤细胞,并在含有二氨基-2-苯基吲哚的Vectashield上载。采用蔡司LSM - 8型高倍显微镜对γ-H2AX核进行观察,平均至少有120个核被计数。γ-H2AX焦点/核的平均值表示DNA双链断裂的数量。 |
| 动物实验 |
CD-1 nude female mice implanted s.c. with HN5 cells, and C.B-17 SCID female mice implanted s.c. with BT474 cells
~100 mg/kg Orally twice daily The C3H/HEN mice were inoculated with a subcutaneous injection of a suspension of MBT-2 cells (100 μl) (1×107 cells/100 μl) into the right flank of the mice on day 1. After one week, the tumor size was measured using vernier calipers, and the volume was calculated. A mean volume of 162 mm3 was regarded as a criterion for tumor establishment. After successful establishment of tumor, the mice were divided into four groups: Group 1, the control group (vehicle treated with 0.5% methylcellulose and 0.1% Tween-80); Group 2, lapatinib -treated (200 mg/kg/day); Group 3, vehicle and irradiation (15 Gy) on day 4; Group 4, lapatinib -treated (200 mg/kg/day) and irradiated (15 Gy) on the 4th day. The body weight of all the mice was recorded every week. Positron emission tomography (PET) and computed tomography (CT) scans were taken (PET/CT) by intravenous injection of the animals with 14 MBq (378 Ci) of fludeoxyglucose (FDG) in saline via the tail vein. [2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorption following oral administration of lapatinib is incomplete and variable. Lapatinib undergoes extensive metabolism, primarily by CYP3A4 and CYP3A5, with minor contributions from CYP2C19 and CYP2C8 to a variety of oxidated metabolites, none of which accounts for more than 14% of the dose recovered in the feces or 10% of lapatinib concentration in plasma. After administration of a single oral dose of (14)C-lapatinib, the predominant route of elimination of drug-related material in the mouse, rat and dog was in the feces, with very little urinary excretion. Most of the dose was eliminated within 48 hours post-dose. Elimination of lapatinib is predominantly through metabolism by CYP3A4/5 with negligible (<2%) renal excretion. Recovery of parent lapatinib in feces accounts for a median of 27% (range 3% to 67%) of an oral dose. Systemic exposure to lapatinib is increased when administered with food. Lapatinib AUC values were approximately 3- and 4-fold higher (Cmax approximately 2.5- and 3-fold higher) when administered with a low-fat (5% fat-500 calories) or with a high-fat (50% fat-1,000 calories) meal, respectively. Lapatinib is highly bound (>99%) to albumin and alpha-1 acid glycoprotein. In vitro studies indicate that lapatinib is a substrate for the transporters breast cancer-resistance protein (BCRP, ABCG2) and P-glycoprotein (P-gp, ABCB1). Lapatinib has also been shown to inhibit P-gp, BCRP, and the hepatic uptake transporter OATP 1B1, in vitro at clinically relevant concentrations. For more Absorption, Distribution and Excretion (Complete) data for Lapatinib (7 total), please visit the HSDB record page. Metabolism / Metabolites Lapatinib undergoes extensive metabolism, primarily by CYP3A4 and CYP3A5, with minor contributions from CYP2C19 and CYP2C8 to a variety of oxidated metabolites, none of which accounts for more than 14% of the dose recovered in the feces or 10% of lapatinib concentration in plasma. Lapatinib, an oral breast cancer drug, has recently been reported to be a mechanism-based inactivator of cytochrome P450 (P450) 3A4 and also an idiosyncratic hepatotoxicant. It was suggested that formation of a reactive quinoneimine metabolite was involved in mechanism-based inactivation (MBI) and/or hepatotoxicity. We investigated the mechanism of MBI of P450 3A4 by lapatinib. Liquid chromatography-mass spectrometry analysis of P450 3A4 after incubation with lapatinib did not show any peak corresponding to irreversible modifications. The enzymatic activity inactivated by lapatinib was completely restored by the addition of potassium ferricyanide. These results indicate that the mechanism of MBI by lapatinib is quasi-irreversible and mediated via metabolic intermediate complex (MI complex) formation. This finding was verified by the increase in a signature Soret absorbance at approximately 455 nm. Two amine oxidation products of the metabolism of lapatinib by P450 3A4 were characterized: N-hydroxy lapatinib (M3) and the oxime form of N-dealkylated lapatinib (M2), suggesting that a nitroso or another related intermediate generated from M3 is involved in MI complex formation. In contrast, P450 3A5 was much less susceptible to MBI by lapatinib via MI complex formation than P450 3A4. In addition, P450 3A5 had a significantly lower ability than 3A4 to generate M3, consistent with N-hydroxylation as the initial step in the pathway to MI complex formation. In conclusion, our results demonstrate that the primary mechanism for MBI of P450 3A4 by lapatinib is not irreversible modification by the quinoneimine metabolite, but quasi-irreversible MI complex formation mediated via oxidation of the secondary amine group of lapatinib. Lapatinib undergoes extensive metabolism in humans to numerous oxidated and N- and O-dealkylated products. In vitro studies using human hepatocytes and microsomes indicated that lapatinib is primarily metabolised by CYP3A4 and CYP3A5, with smaller contributions from CYP2C8. Additional studies indicated that CYP1A2, 2D6, 2C9 and 2C19 may also be involved, but to a lesser extent. The most prominent metabolites are the carboxylic acid GW42393 and the O-dealkylated phenol GW690006. N-oxidation of the secondary aliphatic amine produced a cascade of about 8 minor metabolites. Relative to parent drug, GW690006 produced approximately equipotent inhibition of ErbB1-dependent tumour cell growth in vitro, but was approximately 100-fold less potent in ErbB2- dependent tumour cells. GW342393 was found to be approximately 40-fold less potent than parent drug in both ErbB1- and ErbB2-dependent tumour cells. They are unlikely to contribute to the biological activity of lapatinib. Lapatinib, an oral tyrosine kinase inhibitor used for breast cancer, has been reported to cause idiosyncratic hepatotoxicity. Recently, it has been found that lapatinib forms a metabolite-inhibitor complex (MIC) with CYP3A4 via the formation of an alkylnitroso intermediate. Because CYP3A5 is highly polymorphic compared with CYP3A4 and also oxidizes lapatinib, we investigated the interactions of lapatinib with CYP3A5. Lapatinib inactivated CYP3A5 in a time-, concentration-, and NADPH-dependent manner using testosterone as a probe substrate with K(I) and k(inact) values of 0.0376 mM and 0.0226 min(-1), respectively. However, similar results were not obtained when midazolam was used as the probe substrate, suggesting that inactivation of CYP3A5 by lapatinib is site-specific. Poor recovery of CYP3A5 activity postdialysis and the lack of a Soret peak confirmed that lapatinib does not form a MIC with CYP3A5. The reduced CO difference spectrum further suggested that a large fraction of the reactive metabolite of lapatinib is covalently adducted to the apoprotein of CYP3A5. GSH trapping of a reactive metabolite of lapatinib formed by CYP3A5 confirmed the formation of a quinoneimine-GSH adduct derived from the O-dealkylated metabolite of lapatinib. In silico docking studies supported the preferential formation of an O-dealkylated metabolite of lapatinib by CYP3A5 compared with an N-hydroxylation reaction that is predominantly catalyzed by CYP3A4. In conclusion, lapatinib appears to be a mechanism-based inactivator of CYP3A5 via adduction of a quinoneimine metabolite. Metabolism of lapatinib was assessed both quantitatively and qualitatively in the plasma and excreta of rats (10 mg/kg), dogs (10 mg/kg), mice (30 mg/kg) and humans (250 mg) following a single oral administration of (14)C-lapatinib. In general, (14)C-lapatinib was primarily metabolized, secreted in the bile and eliminated in the feces. In the nonclinical and clinical metabolism studies, urine samples were not analyzed due to the low percentage of the dose excreted by this route. In plasma, (14)C-lapatinib represented the largest single component in all species. Lapatinib was more extensively metabolised in male rats than in female rats, however the metabolic profiles were similar. In dogs and humans, 14C-lapatinib was the only quantifiable peak present. In humans, lapatinib accounted for only approximately half of the radioactivity in the plasma. The remaining radioactivity was attributed to at least 8 metabolites detected by LC-MS but below the limit of radiochemical quantification (approximately 5% of the total radioactivity in pooled plasma). These metabolites were attributed to the N-oxidation cascade that was also observed in vitro as well as in rats and mice. In both mice and rats, only a few of these metabolites were quantifiable in plasma by radiochemical detection, but all were characterized by mass spectrometry. Thus, no unique circulating metabolites were observed in humans Biological Half-Life Single-dose terminal half life: 14.2 hours Effective multiple-dose half life: 24 hours At clinical doses, the terminal phase half-life following a single dose was 14.2 hours; accumulation with repeated dosing indicates an effective half-life of 24 hours. In a mass-balance study, single 250 mg doses of (14)C-labelled lapatinib administered to 6 healthy volunteers produced serum concentrations of radio-labelled material representing parent drug and metabolites that peaked 4 hr after the dose and declined with a median half-life of 6 hr. Plasma concentrations of lapatinib declined with a half-life of 14 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Lapatinib is a yellow solid formulated into film-coated tablets. Lapatinib, an inhibitor of human epidermal growth factor receptor type 2 (HER2/ERBB2) and epidermal growth factor receptor (HER1/EGFR/ERBB1) tyrosine kinases, is an antineoplastic agent. It is used in combination with capecitabine for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress HER2 and who have received prior therapy including an anthracycline, a taxane, and trastuzumab. It is also used in combination with letrozole for the treatment of postmenopausal women with hormone receptor-positive metastatic breast cancer that overexpresses the HER2 receptor, and for whom hormonal therapy is indicated. HUMAN EXPOSURE AND TOXICITY: Asymptomatic and symptomatic cases of overdose have been reported. The doses ranged from 2,500 to 9,000 mg daily, the duration varied between 1 and 17 days. Symptoms observed include lapatinib-associated events and in some cases sore scalp, sinus tachycardia (with otherwise normal ECG), and/or mucosal inflammation. At therapeutic doses, hepatotoxicity, manifested as increases in serum concentrations of aminotransferases and bilirubin, has been observed in clinical trials and post-marketing experience with lapatnib. The hepatotoxicity may be severe and deaths have been reported. Causality of the deaths is uncertain. The hepatotoxicity may occur within days to several months after initiation of treatment. Women should avoid the use of lapatinib during pregnancy. While there are not adequate and well-controlled studies in pregnant women, lapatinib has been associated with adverse reproductive effects in animals. If used during pregnancy, the patient should be apprised of the potential fetal hazard. ANIMAL STUDIES: While there was no evidence of carcinogenicity in a two year mouse study, increased mortality which was related to skin toxicities was observed in males at 150 and 300 mg/kg/day and in females at 300 mg/kg/day. In a two-year rat carcinogenicity study, increased mortality was observed in males at 500 mg/kg/day and females at 300 mg/kg/day, and was related to skin toxicities. Renal infarcts and papillary necrosis were observed in females from 60 mg/kg/day and 180 mg/kg/day, respectively. An increased incidence of benign hemangioma of the mesenteric lymph nodes was noted in males from 120 mg/kg/day and in females at 180 mg/kg/day but was within background range. The clinical significance of these findings to humans is not known. Lapatinib did not affect male or female rat gonadal function, mating, or fertility at doses up to 120 mg/kg/day in females and up to 180 mg/kg/day in males. Studies in pregnant rats and rabbits revealed no teratogenic effects. However, in rats, minor anomalies (left-sided umbilical artery, cervical rib and precocious ossification) occurred at the maternally toxic dose of 120 mg/kg/day. In rabbits, lapatinib was associated with maternal toxicity at 60 and 120 mg/kg/day and abortions at 120 mg/kg/day. At maternally toxic doses, decreased fetal body weights, decreased number of live fetuses and minor skeletal variations were noted. Lapatinib was not clastogenic or mutagenic in a battery of assays including the Chinese hamster chromosome aberration assay, the Ames assay, human lymphocyte chromosome aberration assay and an in vivo rat bone marrow chromosome aberration assay. Hepatotoxicity Elevations in serum aminotransferase levels are common during lapatinib therapy, occurring in up to half of patients. Values greater than 5 times the upper limit of normal (ULN) occur in 2% to 6% of patients but are usually transient and asymptomatic. Dose adjustments or temporary discontinuations are rarely required for liver test abnormalities. Since its introduction into clinical use, lapatinib has been linked to several cases of clinically apparent acute liver injury. The clinical features of injury have not been well defined, but the onset is usually within 1 to 3 months of starting lapatinib and the pattern of serum enzyme elevations is typically hepatocellular or mixed (Case 1). Sufficent numbers of reports of liver injury have been made to the Food and Drug Administration such that lapatinib is listed as having hepatotoxicity that can be fatal. The frequency of serious liver injury is estimated to be 0.2%, but is likely higher. Immunoallergic and autoimmune features are uncommon, although genetic studies suggest that lapatinib hepatotoxicity is linked to specific HLA alleles. Most instances are self-limited, but several cases of acute liver failure have been reported with tyrosine kinase receptor inhibitors including imatinib, sunitinib, lapatinib, gefitinib and erlotinib. Recurrence of injury is common with reexposure but may not occur upon switching to another kinase receptor inhibitor. Likelihood score: B (likely cause of clinically apparent acute liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of lapatinib during breastfeeding. Because lapatinib is more than 99% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life is about 24 hours and it might accumulate in the infant. It is also given in combination with capecitabine, which may increase the risk to the infant. The manufacturer recommends that breastfeeding be discontinued during lapatinib therapy and for 1 week after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Highly bound (>99%) to albumin and alpha-1 acid glycoprotein Interactions Grapefruit products should be avoided because of the potential for increased plasma lapatinib concentrations. In patients receiving lapatinib and paclitaxel (a CYP2C8 and P-gp substrate) concomitantly, systemic exposure (AUC over 24 hours) of paclitaxel was increased by 23%. However, the manufacturer states that because of limitations of the study design, these data may underestimate the potential increase in paclitaxel exposure during concomitant use. Concomitant administration of lapatinib and oral digoxin (a P-gp substrate) increased systemic exposure (AUC) of digoxin by approximately 2.8-fold. In patients receiving digoxin, serum digoxin concentrations should be measured prior to initiation of lapatinib therapy and monitored throughout concomitant therapy. If serum digoxin concentrations exceed 1.2 ng/mL, digoxin dosage should be reduced by 50%. Because lapatinib may cause prolongation of the QT interval, lapatinib should be used with caution in patients receiving concomitant therapy with other drugs (e.g., antiarrhythmic agents) known to prolong the QT interval. For more Interactions (Complete) data for Lapatinib (10 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
A quinazoline derivative that inhibits EPIDERMAL GROWTH FACTOR RECEPTOR and HER2 (RECEPTOR, ERBB-2) tyrosine kinases. It is used for the treatment of advanced or metastatic breast cancer, where tumors overexpress HER2.
See also: Lapatinib Ditosylate (annotation moved to). Therapeutic Uses Antineoplastic Agents; Protein Kinase Inhibitors Tykerb is indicated in combination with capecitabine for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress HER2 and who have received prior therapy including an anthracycline, a taxane, and trastuzumab. Limitation of Use: Patients should have disease progression on trastuzumab prior to initiation of treatment with Tykerb in combination with capecitabine. /Included in US product label/ Tykerb is indicated in combination with letrozole for the treatment of postmenopausal women with hormone receptor-positive metastatic breast cancer that overexpresses the HER2 receptor for whom hormonal therapy is indicated. /Included in US product label/ EXPL THER Although there are effective HER2-targeted agents, novel combination strategies in HER2-overexpressing breast cancers are needed for patients whose tumors develop drug resistance. To develop new therapeutic strategy, ... the combinational effect of entinostat, an oral isoform-selective histone deacetylase type I inhibitor, and lapatinib, a HER2/EGFR dual tyrosine kinase inhibitor, in HER2+ breast cancer cells /was investigated/. ... The combinational synergistic effect and its mechanism by CellTiter Blue assay, flow cytometry, anchorage-independent growth, quantitative real-time PCR, small interfering RNA, Western blotting, and mammary fat pad xenograft mouse models /was assessed/. /It was/ found that compared with entinostat or lapatinib alone, the two drugs in combination synergistically inhibited proliferation (P < 0.001), reduced in vitro colony formation (P < 0.05), and resulted in significant in vivo tumor shrinkage or growth inhibition in two xenograft mouse models (BT474 and SUM190, P < 0.001). The synergistic anti-tumor activity of the entinostat/lapatinib combination was due to downregulation of phosphorylated Akt, which activated transcriptional activity of FOXO3, resulting in induction of Bim1 (a BH3 domain-containing pro-apoptotic protein). Furthermore, entinostat sensitized trastuzumab/lapatinib-resistance-HER2-overexpressing cells to the trastuzumab/lapatinib combination and enhanced the anti-proliferation effect compare with single or double combination treatment. This study provides evidence that entinostat has enhanced anti-tumor effect in combination with HER2-targeted reagent, lapatinib, and resulting in induction of apoptosis by FOXO3-mediated Bim1 expression. ... Findings justifies for conducting a clinical trial of combinational treatment with entinostat, lapatinib, and trastuzumab in patients with HER2-overexpressing breast cancer resistant to trastuzumab-based treatment. Drug Warnings /BOXED WARNING/ WARNING: HEPATOTOXICITY. Hepatotoxicity has been observed in clinical trials and postmarketing experience. The hepatotoxicity may be severe and deaths have been reported. Causality of the deaths is uncertain. Hepatotoxicity (ALT or AST >3 times the upper limit of normal and total bilirubin >2 times the upper limit of normal) has been observed in clinical trials (<1% of patients) and postmarketing experience. The hepatotoxicity may be severe and deaths have been reported. Causality of the deaths is uncertain. The hepatotoxicity may occur days to several months after initiation of treatment. Liver function tests (transaminases, bilirubin, and alkaline phosphatase) should be monitored before initiation of treatment, every 4 to 6 weeks during treatment, and as clinically indicated. If changes in liver function are severe, therapy with Tykerb should be discontinued and patients should not be retreated with Tykerb. Lapatinib may cause fetal harm; fetal anomalies, abortion, and death of offspring within days after birth have been demonstrated in animals. Pregnancy should be avoided during therapy. If lapatinib is used during pregnancy or if the patient becomes pregnant while receiving the drug, the patient should be apprised of the potential hazard to the fetus. FDA Pregnancy Risk Category: D /POSITIVE EVIDENCE OF RISK. Studies in humans, or investigational or post-marketing data, have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective./ For more Drug Warnings (Complete) data for Lapatinib (14 total), please visit the HSDB record page. Pharmacodynamics Lapatinib is a small molecule and a member of the 4-anilinoquinazoline class of kinase inhibitors. An anti-cancer drug, lapatinib was developed by GlaxoSmithKline (GSK) as a treatment for solid tumours such as breast and lung cancer. It was approved by the FDA on March 13, 2007, for use in patients with advanced metastatic breast cancer in conjunction with the chemotherapy drug capecitabine. The epidermal growth factor receptor (EGFR) and ErbB-2 transmembrane tyrosine kinases are currently being targeted by various mechanisms in the treatment of cancer. GW2016 is a potent inhibitor of the ErbB-2 and EGFR tyrosine kinase domains with IC50 values against purified EGFR and ErbB-2 of 10.2 and 9.8 nM, respectively. This report describes the efficacy in cell growth assays of GW2016 on human tumor cell lines overexpressing either EGFR or ErbB-2: HN5 (head and neck), A-431 (vulva), BT474 (breast), CaLu-3 (lung), and N87 (gastric). Normal human foreskin fibroblasts, nontumorigenic epithelial cells (HB4a), and nonoverexpressing tumor cells (MCF-7 and T47D) were tested as negative controls. After 3 days of compound exposure, average IC50 values for growth inhibition in the EGFR- and ErbB-2-overexpressing tumor cell lines were < 0.16 microM. The average selectivity for the tumor cells versus the human foreskin fibroblast cell line was 100-fold. Inhibition of EGFR and ErbB-2 receptor autophosphorylation and phosphorylation of the downstream modulator, AKT, was verified by Western blot analysis in the BT474 and HN5 cell lines. As a measure of cytotoxicity versus growth arrest, the HN5 and BT474 cells were assessed in an outgrowth assay after a transient exposure to GW2016. The cells were treated for 3 days in five concentrations of GW2016, and cell growth was monitored for an additional 12 days after removal of the compound. In each of these tumor cell lines, concentrations of GW2016 were reached where outgrowth did not occur. Furthermore, growth arrest and cell death were observed in parallel experiments, as determined by bromodeoxyuridine incorporation and propidium iodide staining. GW2016 treatment inhibited tumor xenograft growth of the HN5 and BT474 cells in a dose-responsive manner at 30 and 100 mg/kg orally, twice daily, with complete inhibition of tumor growth at the higher dose. Together, these results indicate that GW2016 achieves excellent potency on tumor cells with selectivity for tumor versus normal cells and suggest that GW2016 has value as a therapy for patients with tumors overexpressing either EGFR or ErbB-2. [1] Background The aim of this study was to evaluate the effect of lapatinib, a dual inhibitor of epidermal growth factor receptor (EGFR) and HER-2, on the radiosensitivity of murine bladder tumor line-2 (MBT-2) cells in vitro and in vivo. Material/Methods MBT-2 cells were pretreated with lapatinib at doses ranging from 200–1,000 nM for 30 min followed by radiation at doses ranging from 2.5–10 Gy for 30 min. A clonogenic assay (colony formation assay) assessed cell survival. Western blot measured phosphorylated epidermal growth factor receptor (p-EGFR), phosphorylated AKT (p-AKT), and phosphorylated HER-2 (p-HER2) and the apoptosis marker, PARP. The C3H/HeN mouse tumor xenograft model underwent subcutaneous injection of MBT-2 cells; mice were divided into four groups, treated with lapatinib (200 mg/kg), radiation (15 Gy), a combination of both, and with vehicle (control). Results Lapatinib pretreatment, combined with radiation, decreased MBT-2 cell survival, and suppressed radiation-activated levels of p-EGFR and p-HER-2. MBT-2 cells treated with a 10 Gy dose of radiation and 1000 nM of lapatinib showed combination index (CI) values of <1 indicating synergy. Increased expression of γ-H2AX, indicated increased apoptosis. In mice with tumor xenografts, a daily dose of lapatinib (200 mg/kg/day) for seven days combined with radiation on the fourth day suppressed tumor growth to a greater degree than radiation alone. Conclusions Lapatinib treatment enhanced the radiation sensitivity in an in vitro and in vivo murine bladder cancer model by decreasing radiation-mediated EGFR and HER-2 activation, and by causing DNA damage leading to cell apoptosis. In the mid-1980s, it was recognized that overexpression of epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor-2 (HER2) adversely affected the prognosis of some cancer patients. Because EGFR and HER2 are key regulators of cell growth, differentiation, and survival, it was believed that inhibition of these receptors would block downstream signaling and would be antiproliferative. Indeed, by the late 1980s, researchers were creating some of the first targeted inhibitors of tyrosine kinase activity. It was in this context that we began our efforts to develop potential disease therapies using a small-molecule approach to selectively target EGFR and HER2. The challenge for developing small-molecule EGFR/HER2 inhibitors lay in determining the potency and selectivity specific to their kinase domains. Several key components of our study permitted us to drive discovery efforts. First, novel chemistry produced numerous compounds for testing. Second, a broad-based kinase biochemical screening platform allowed us to examine compounds on multiple kinase targets. Third, the creation of a cell-based panel included lines dependent on EGFR or HER2 signaling as well as suitable control cell lines. This cell panel not only provided automated evaluation of molecules for potency and selectivity in the cell's complex environment but also made the investigation of players downstream of EGFR and HER2 possible, linking EGFR and HER2 inhibition to cell-cycle arrest and apoptosis. The last component, in vivo xenograft models, enabled us to continue this research by using pharmacokinetic and pharmacodynamic markers. Our work with a highly committed and motivated team of scientists ultimately resulted in the innovative discovery of GW2016, also known as GW572016, which later became the cancer therapy lapatinib. The selectivity of lapatinib for HER2 and EGFR kinase domains and its activity in HER2-overexpressing cell lines (e.g., breast and gastric cancer) and EGFR-overexpressing cell lines (e.g., head and neck cancer) provided a foundation to test lapatinib in selected patient populations. The U.S. Food and Drug Administration's approval in 2007 of lapatinib, in combination with capecitabine for treatment of advanced or metastatic HER2-overexpressing breast cancer, provided another option for patients whose disease progressed on trastuzumab (a humanized monoclonal antibody directed against the extracellular domain of HER2). Ongoing clinical trials are examining lapatinib activity in HER2-overexpressing breast cancer, HER2-overexpressing gastric cancer, and head and neck cancer. Looking ahead, an HER2- and EGFR-targeted strategy will be similar to most future cancer therapies (i.e., in combination with other agents). Recent clinical evidence has shown the synergy of dual blockade with the combination of lapatinib and trastuzumab in patients with HER2-positive metastatic breast cancer. In addition, dual blockade of the HER2-signaling pathway is also being examined in the neoadjuvant and adjuvant settings. Other clinical studies are evaluating HER2 and EGFR agents in combination with other signaling agents and chemotherapies. Since our first publication on lapatinib 10 years ago, we have been excited about the impact of our efforts, and we remain committed to improving the lives of cancer patients. https://aacrjournals.org/mct/article/10/11/2019/90946/The-Discovery-of-Lapatinib-GW572016-Commentary-on |
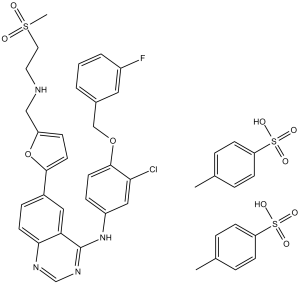
| 分子式 |
C43H42CLFN4O10S3
|
|---|---|
| 分子量 |
925.46
|
| 精确质量 |
924.173
|
| 元素分析 |
C, 55.81; H, 4.57; Cl, 3.83; F, 2.05; N, 6.05; O, 17.29; S, 10.39
|
| CAS号 |
388082-77-7
|
| 相关CAS号 |
Lapatinib;231277-92-2;Lapatinib ditosylate monohydrate;388082-78-8;Lapatinib tosylate;1187538-35-7; Lapatinib ditosylate;388082-77-7; Lapatinib-d4;1184263-99-7; Lapatinib-d7 dihydrochloride;Lapatinib-d5;2748212-14-6;Lapatinib-d4-1;1184264-15-0
|
| PubChem CID |
9941095
|
| 外观&性状 |
Yellow solid powder
|
| 沸点 |
750.7ºC at 760 mmHg
|
| 熔点 |
240-242ºC
|
| 闪点 |
407.8ºC
|
| LogP |
12.328
|
| tPSA |
240.23
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
15
|
| 可旋转键数目(RBC) |
13
|
| 重原子数目 |
62
|
| 分子复杂度/Complexity |
1100
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=S(CCNCC1=CC=C(C2=CC=C3C(C(NC4=CC(Cl)=C(C=C4)OCC5=CC(F)=CC=C5)=NC=N3)=C2)O1)(C)=O.O=S(C6=CC=C(C)C=C6)(O)=O.O=S(C7=CC=C(C)C=C7)(O)=O
|
| InChi Key |
UWYXLGUQQFPJRI-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C29H26ClFN4O4S.2C7H8O3S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19;2*1-6-2-4-7(5-3-6)11(8,9)10/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35);2*2-5H,1H3,(H,8,9,10)
|
| 化学名 |
N-[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]-6-[5-[(2-methylsulfonylethylamino)methyl]furan-2-yl]quinazolin-4-amine;4-methylbenzenesulfonic acid
|
| 别名 |
GSK 572016; Trade name: Tykerb.GW2016; GSK 572016 ditosylate; GW-2016 ditosylate; GSK-572016 ditosylate; GSK-572016; GW-2016; GSK572016 ditosylate; GW2016 ditosylate; GW 2016; Lapatinib ditosylate; SK572016; GW 2016; Lapatinib
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.33 mg/mL (2.52 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 23.3 mg/mL的澄清DMSO储备液加入到400 μL PEG300中并混合均匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.33 mg/mL (2.52 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 23.3 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: 2% DMSO+30% PEG 300+ddH2O: 10 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.0805 mL | 5.4027 mL | 10.8054 mL | |
| 5 mM | 0.2161 mL | 1.0805 mL | 2.1611 mL | |
| 10 mM | 0.1081 mL | 0.5403 mL | 1.0805 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02101905 | Active Recruiting |
Drug: Lapatinib Drug: Lapatinib Ditosylate |
Gliosarcoma Mixed Glioma |
National Cancer Institute (NCI) |
March 13, 2014 | Phase 1 |
| NCT01273610 | Active Recruiting |
Drug: Lapatinib Drug: Trastuzumab |
Breast Neoplasms HER2/Neu Positive |
City of Hope Medical Center | April 20, 2011 | Phase 2 |
| NCT00999804 | Active Recruiting |
Drug: Lapatinib Drug: Letrozole |
Breast Cancer | Baylor Breast Care Center | October 2011 | Phase 2 |
| NCT00680901 | Active Recruiting |
Drug: Lapatinib Drug: Placebo |
Neoplasms, Gastrointestinal Tract | Novartis Pharmaceuticals | June 4, 2008 | Phase 3 |
| NCT00470704 | Active Recruiting |
Drug: Lapatinib Drug: Herceptin |
Breast Cancer | Nancy Lin, MD | May 14, 2007 | Phase 2 |
|
 |