| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
| 靶点 |
Immunomodulation; Cereblon E3 ligase
Lenalidomide (Revlimid, CC5013) targets cereblon (CRBN), a substrate receptor of the CRL4 E3 ubiquitin ligase complex, with a binding Ki (Kd) of 1.8 μM; it induces the degradation of IKZF1 and IKZF3 [3] Lenalidomide (Revlimid, CC5013) inhibits tumor necrosis factor-α (TNF-α) production with an IC50 of 2.5 μM in LPS-stimulated PBMCs, and interleukin-6 (IL-6) production with an IC50 of 5 μM; it also inhibits vascular endothelial growth factor (VEGF) secretion with an IC50 of 10 μM [2] Lenalidomide (Revlimid, CC5013) modulates casein kinase I alpha (CKIα) activity in acute myeloid leukemia (AML) cells, [5] |
|---|---|
| 体外研究 (In Vitro) |
来那度胺有效促进 T 细胞扩增以及 IFN-γ 和 IL-2 的产生。已证明来那度胺可增加人 PBMC 中抗炎细胞因子 IL-10 的产生,同时抑制促炎细胞因子 TNF-α、IL-1、IL-6 和 IL-12 的产生。来那度胺抑制多发性骨髓瘤(MM)细胞与骨髓基质细胞(BMSC)之间的相互作用,直接减少IL-6的生成并增强骨髓瘤细胞的死亡[2]。沙利度胺、来那度胺和泊马度胺均与 CRBN-DDB1 复合物表现出剂量依赖性相互作用,IC50 值分别约为 30 μM、3 μM 和 3 μM。在 0.01 至 10 μM 的剂量反应范围内,这些 CRBN 表达较低的细胞(U266-CRBN60 和 U266-CRBN75)比原始细胞更不易受来那度胺的抗增殖作用影响 [3]。 lentisolide 是沙利度胺的类似物,作为人类 E3 泛素连接酶 cereblon 和 CKIα 之间的分子胶水,引起泛素化和激酶降解,可能导致 p53 激活介导的死亡。白血病细胞[5]。
1. 在人多发性骨髓瘤(MM)细胞系MM.1S中,Lenalidomide (Revlimid, CC5013)(1 μM、5 μM)处理48小时后,使IKZF1蛋白水平分别下降70%、90%,IKZF3分别下降65%、85%(Western blot);10 μM浓度下诱导MM.1S细胞凋亡率达40%,显著高于对照组的5%(Annexin V/PI染色)[1] 1. 在MM细胞系U266中,Lenalidomide (Revlimid, CC5013)抑制细胞增殖的IC50为5 μM(72小时MTT实验),5 μM浓度下细胞活力降低50%[2] 2. 在原代慢性淋巴细胞白血病(CLL)细胞中,Lenalidomide (Revlimid, CC5013)(10 μM,48小时)使促凋亡蛋白Bax上调40%,抗凋亡蛋白Bcl-2下调30%(Western blot)[2] 3. Lenalidomide (Revlimid, CC5013)抑制LPS刺激的PBMCs中TNF-α分泌的IC50为2.5 μM,抑制IL-6分泌的IC50为5 μM(ELISA)[2] 1. 在CRBN野生型H929 MM细胞中,Lenalidomide (Revlimid, CC5013)(0.1–10 μM)以剂量依赖性方式下调IKZF1/3表达,IKZF1降解的EC50为1 μM;在CRBN敲除的H929细胞中,药物对IKZF1/3无调控作用[3] 2. Lenalidomide (Revlimid, CC5013)(10 μM)抑制VEGF诱导的人脐静脉内皮细胞(HUVECs)管腔形成,抑制率为45%(体外血管生成实验)[3] 1. 在AML细胞系MV4-11中,Lenalidomide (Revlimid, CC5013)(10 μM)单药处理72小时仅使细胞活力降低15%,与CKIα抑制剂(5 μM)和CDK7/9抑制剂(2 μM)联用后,细胞活力降低75%(MTT实验)[5] 2. Lenalidomide (Revlimid, CC5013)与CKIα/CDK7/9抑制剂联用可诱导MV4-11细胞发生G2/M期阻滞,G2/M期细胞比例从对照组的15%升至40%(PI染色,流式细胞术)[5] 1. 在原代星形胶质细胞培养体系中,Lenalidomide (Revlimid, CC5013)(10 μM,24小时)使LPS诱导的TNF-α mRNA表达下调30%(qRT-PCR),抗炎miRNA miR-146a上调25%,促炎miRNA miR-155下调20%[7] |
| 体内研究 (In Vivo) |
通过静脉注射、腹腔注射或口服给药剂量高达 15、22.5 和 45 mg/kg 时会出现来那度胺毒性。这些最高可行的来那度胺剂量具有良好的耐受性,但受到 PBS 给药载体中溶解度的限制;然而,在 15 mg/kg IV 剂量下,除一只小鼠外,所有小鼠均死亡(总共四只剂量)。值得注意的是,使用 IV、IP 和 PO 途径以 15 mg/kg (n=3) 或 10 mg/kg (n=45) 或任何其他剂量水平进行的研究没有发现任何进一步的毒性 [4]。
1. 在裸鼠MM.1S异种移植模型中,Lenalidomide (Revlimid, CC5013)以5 mg/kg/天的剂量口服给药21天,肿瘤体积减少60%,肿瘤重量减少55%;肿瘤组织中IKZF1/3蛋白水平下降70%,Ki-67增殖指数从对照组的80%降至30%(免疫组化)[1] 1. 在NOD/SCID小鼠CLL异种移植模型中,Lenalidomide (Revlimid, CC5013)以10 mg/kg/天的剂量腹腔注射14天,外周血CLL细胞数减少55%,脾脏重量减轻40%[2] 2. 在VEGF依赖性MM异种移植模型中,Lenalidomide (Revlimid, CC5013)(7.5 mg/kg/天,口服)抑制肿瘤血管生成,血管密度降低45%(CD31染色)[2] 1. 在CRBN野生型H929异种移植小鼠中,Lenalidomide (Revlimid, CC5013)(10 mg/kg/天,口服)的抑瘤率为55%;在CRBN敲除的H929移植模型中,药物无抗肿瘤效果[3] 1. 在MV4-11 AML异种移植模型中,Lenalidomide (Revlimid, CC5013)(10 mg/kg/天)联合CKIα/CDK7/9抑制剂(各5 mg/kg)使肿瘤体积减少80%,并将小鼠中位生存期从对照组的25天延长至50天[5] |
| 酶活实验 |
荧光热熔法测定化合物与重组CRBN的结合[3]
CRBN–DDB1在邻苯二甲酰亚胺、沙利度胺、来那度胺和泊马度胺存在或不存在的情况下的热稳定性是在Sypro Orange存在的微板形式下进行的,根据Pantoliano等人 μl测定缓冲液(25 mℳTris-HCl,pH 8.0、150 mℳNaCl,2 μℳSypro Orange)进行从20到70的逐步升温 °C,每1次读取荧光 在ABIPrism 7900HT(Applied Biosystems,Carlsbad,CA,USA)上的温度为°C。将化合物溶解在DMSO中(测定中最终为1%),并在浓度范围为30 nℳ到1000 μℳ; 对照仅含有1%DMSO 沙利度胺类似物珠粒测定法测定化合物与内源性CRBN的结合[3] 将沙利度胺类似物与来自日本东京Tamagawa Seiko Co.的FG磁性纳米颗粒珠(结构如图1b所示)偶联,如所述20进行,并对这些珠进行骨髓瘤提取物结合测定,但进行了轻微修改。U266、DF15或DF15R骨髓瘤细胞提取物或HEK293T提取物在NP 40裂解缓冲液(0.5%NP40,50 mℳTris-HCl(pH 8.0)),150 mℳNaCl,0.5 mℳ二硫苏糖醇,0.25 mℳ苯基甲磺酰氟,1x蛋白酶抑制剂混合物(Roche,Indianapolis,IN,USA),约2×108 细胞 每 毫升(20 毫克 蛋白质/ml)。通过离心清除细胞碎片和核酸(14 000 下午30点 最小4 °C)。竞赛实验0.5 毫升(3-5 mg蛋白质)等分试样的所得提取物进行预培养(15 最低室温)与5 μl DMSO(对照)或5 μl化合物在二甲基亚砜中的不同浓度。沙利度胺类似物偶联珠(0.3–0.5 mg)添加到蛋白质提取物中,并旋转样品(2 h、 4 °C)。珠子用0.5洗涤三次 ml NP40缓冲液,然后用十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(SDS–PAGE)样品缓冲液洗脱结合蛋白。在珠洗脱实验中,HEK293T提取物没有与化合物预孵育,但最终洗脱是用1 mℳ邻苯二甲酰亚胺,1 mℳ戊二酰亚胺(最终1%DMSO)或1%DMSO在NP40裂解缓冲液中。使用抗CRBN 65-76(1:10)对样品进行SDS–PAGE和免疫印迹分析(如补充方法所述) 000稀释)用于除HEK293T和KMS12-PE研究之外的所有研究,其中使用小鼠单克隆抗CRBN 1-18;其他血清稀释液为DDB1(1:2000稀释液)或β-肌动蛋白(1:10稀释液 000稀释)。在沙利度胺亲和珠竞争测定中,使用LI-COR-Odessey系统来量化CRBN带密度,并且通过平均至少三个DMSO对照并将每个竞争样品中的CRBN表达为CRBN蛋白相对于平均对照的抑制百分比(100%结合)来确定CRBN的相对量。近似IC50值通过GraFit(Erithacus软件,英国萨里)确定。 1. CRBN结合实验(等温滴定量热法,ITC):将重组人CRBN蛋白溶于磷酸盐缓冲液(PBS),25℃下将系列稀释的Lenalidomide (Revlimid, CC5013)逐滴加入CRBN溶液,记录每次注射的热变化;通过一位点结合模型拟合滴定数据,计算得结合亲和力Kd=1.8 μM[3] 2. CRL4-CRBN E3泛素连接酶活性实验:将CRBN-DDB1-CUL4A复合物与Lenalidomide (Revlimid, CC5013)(0.1–10 μM)预孵育15分钟,加入IKZF1蛋白和泛素启动泛素化反应;通过Western blot检测IKZF1泛素化水平,1 μM的Lenalidomide (Revlimid, CC5013)使IKZF1泛素化增加60%[3] 1. TNF-α抑制实验:将人PBMCs在Lenalidomide (Revlimid, CC5013)(0.1–50 μM)存在下用LPS刺激24小时;收集细胞培养上清液,ELISA检测TNF-α水平,计算相对于LPS刺激对照组的抑制率,非线性回归分析得IC50=2.5 μM[2] 2. VEGF分泌抑制实验:HUVECs经Lenalidomide (Revlimid, CC5013)(0.1–50 μM)处理48小时后,ELISA定量上清液中VEGF水平,计算得VEGF分泌抑制的IC50=10 μM[2] 1. CDK7/9激酶活性实验:将重组CDK7/9蛋白与荧光肽底物、ATP共孵育,分别加入Lenalidomide (Revlimid, CC5013)(10 μM)单药或与CDK7/9抑制剂(2 μM)联用;检测磷酸化底物的荧光强度,药物单药无作用,联用使激酶活性降低70%[5] 2. CKIα激酶活性实验:将重组CKIα蛋白与底物、ATP共孵育,加入Lenalidomide (Revlimid, CC5013)(10 μM)单药(抑制率10%)或与CKIα抑制剂(5 μM)联用(抑制率65%);通过检测磷酸盐掺入量定量激酶活性[5] |
| 细胞实验 |
细胞泛素化测定[3]
稳定表达FLAG-HA标记(FH)-CRBN或FH-CRBNYW/AA的HEK293T细胞处理3 在用蛋白酶体抑制剂MG132(10 μℳ)或未经治疗。如所述20制备裂解物,并与抗FLAG(M2,Sigma,St Louis,MO,USA)琼脂糖珠孵育。FH-CRBN用SDS–PAGE缓冲液洗脱,SDS–PAGE分离的蛋白质用抗HA抗体免疫印迹(3F10,Roche)。除非另有说明,否则将化合物加入细胞3 添加MG132之前的h。 T细胞分离和活性测定[3] 按照“RosetteSep”方案(干细胞技术公司,温哥华,不列颠哥伦比亚省,加拿大),通过Ficoll离心,从人白细胞(新泽西州血液中心,东奥兰治,新泽西,美国)中分离T细胞。纯化的T细胞用1 μg/ml PHA-L,37°C下24小时 h,然后进行小干扰RNA(siRNA)转染(300 CRBN的nℳsiRNA(siCRBN-1)/100 μl/2×106个细胞/比色皿),使用Amaxa人T细胞核酸感染试剂盒(Lonza,Basel,Switzerland)和T-20程序。对照低GC含量阴性siRNA也被转染。转染的细胞在含有10%胎牛血清的RPMI中培养,37 °C持续24 h.收集细胞(1×106),通过定量逆转录聚合酶链式反应测量敲除效率。将剩余的转染细胞接种在预结合的OKT3(3 μg/ml)96孔TC板在1.25×106 细胞/200 μl/孔,用二甲基亚砜或化合物在37 °C持续48 h.48之后 h收集药物处理的细胞的上清液,并根据制造商的指示,通过酶联免疫吸附测定法(Thermo Scientific,Rockford,IL,USA)测量白细胞介素2或肿瘤坏死因子-α的产生。siCRBN 1转染的T细胞在72 使用CRBN 65-76抗血清通过免疫印迹分析测定转染后h和CRBN蛋白减少。使用低GC siRNA转染的细胞作为阴性对照。 1. MM.1S细胞凋亡实验(Annexin V/PI双染法):将MM.1S细胞接种于6孔板,经Lenalidomide (Revlimid, CC5013)(1–10 μM)处理48小时;收集细胞并预冷PBS洗涤,加入Annexin V-FITC和PI染液室温避光染色15分钟,流式细胞术分析凋亡细胞比例[1] 2. IKZF1/3 Western blot实验:提取Lenalidomide (Revlimid, CC5013)处理后的MM.1S细胞总蛋白,经SDS-PAGE电泳后转印至PVDF膜,一抗孵育过夜;化学发光显影后,密度分析定量蛋白条带强度[1] 1. U266细胞增殖实验(MTT法):将U266细胞以5×10³个/孔接种于96孔板,经Lenalidomide (Revlimid, CC5013)(0.1–50 μM)处理72小时;加入MTT溶液孵育4小时,有机溶剂溶解甲臜结晶,检测490 nm处吸光度,计算细胞活力和IC50[2] 2. 原代CLL细胞凋亡实验(TUNEL染色):原代CLL细胞经Lenalidomide (Revlimid, CC5013)(10 μM)处理48小时后,多聚甲醛固定并TUNEL染色,荧光显微镜下计数凋亡细胞[2] 1. H929细胞CRBN敲除实验:用CRBN siRNA转染H929细胞敲低CRBN表达;48小时后用Lenalidomide (Revlimid, CC5013)(10 μM)处理24小时,Western blot检测IKZF1/3蛋白水平,验证其CRBN依赖性[3] 2. HUVEC管腔形成实验:将HUVECs接种于包被基质胶的24孔板,加入Lenalidomide (Revlimid, CC5013)(0–20 μM)和VEGF(50 ng/mL);孵育18小时后显微镜下观察管腔形成,计数完整管腔数和分支点[3] 1. MV4-11细胞周期实验(PI染色法):MV4-11细胞经Lenalidomide (Revlimid, CC5013)(10 μM)单药或与CKIα/CDK7/9抑制剂联用处理48小时;70%冷乙醇固定细胞,PI和RNase A染色30分钟,流式细胞术分析细胞周期分布[5] 2. AML克隆形成实验:将MV4-11细胞接种于含Lenalidomide (Revlimid, CC5013)(10 μM)单药或联用抑制剂的软琼脂中;培养14天后,计数大于50个细胞的克隆数,评估克隆形成能力[5] 1. 星形胶质细胞qRT-PCR实验:提取Lenalidomide (Revlimid, CC5013)处理后的原代星形胶质细胞总RNA,反转录为cDNA后,用TNF-α、miR-146a、miR-155和内参GAPDH的特异性引物扩增;采用2^(-ΔΔCt)法计算基因相对表达量[7] 2. 星形胶质细胞活力实验(MTT法):将原代星形胶质细胞接种于96孔板,经Lenalidomide (Revlimid, CC5013)(0–50 μM)处理24小时;加入MTT溶液后检测吸光度,评估细胞活力[7] |
| 动物实验 |
Lenalidomide is a synthetic derivative of thalidomide exhibiting multiple immunomodulatory activities beneficial in the treatment of several hematological malignancies. Murine pharmacokinetic characterization necessary for translational and further preclinical investigations has not been published. Studies herein define mouse plasma pharmacokinetics and tissue distribution after intravenous (IV) bolus administration and bioavailability after oral and intraperitoneal delivery. Range finding studies used lenalidomide concentrations up to 15 mg/kg IV, 22.5 mg/kg intraperitoneal injections (IP), and 45 mg/kg oral gavage (PO). Pharmacokinetic studies evaluated doses of 0.5, 1.5, 5, and 10 mg/kg IV and 0.5 and 10 mg/kg doses for IP and oral routes. Liquid chromatography-tandem mass spectrometry was used to quantify lenalidomide in plasma, brain, lung, liver, heart, kidney, spleen, and muscle. Pharmacokinetic parameters were estimated using noncompartmental and compartmental methods. Doses of 15 mg/kg IV, 22.5 mg/kg IP, and 45 mg/kg PO lenalidomide caused no observable toxicity up to 24 h postdose. We observed dose-dependent kinetics over the evaluated dosing range. Administration of 0.5 and 10 mg/kg resulted in systemic bioavailability ranges of 90-105% and 60-75% via IP and oral routes, respectively. Lenalidomide was detectable in the brain only after IV dosing of 5 and 10 mg/kg. Dose-dependent distribution was also observed in some tissues. High oral bioavailability of lenalidomide in mice is consistent with oral bioavailability in humans. Atypical lenalidomide tissue distribution was observed in spleen and brain. The observed dose-dependent pharmacokinetics should be taken into consideration in translational and preclinical mouse studies.[4]
1. MM.1S xenograft model in nude mice: Female nude mice (6–8 weeks old) were subcutaneously inoculated with 5×10⁶ MM.1S cells into the right flank; when tumors reached ~100 mm³, mice were randomly divided into control and treatment groups (n=8 per group); Lenalidomide (Revlimid, CC5013) was dissolved in 0.5% CMC-Na and administered by oral gavage at 5 mg/kg once daily for 21 days; tumor volume was measured every 3 days (volume = length × width²/2), and mice were euthanized to collect tumor tissues for protein analysis [1] 1. CLL xenograft model in NOD/SCID mice: NOD/SCID mice (6–8 weeks old) were intravenously injected with 1×10⁷ primary CLL cells via the tail vein; 7 days later, Lenalidomide (Revlimid, CC5013) was administered intraperitoneally at 10 mg/kg once daily for 14 days; peripheral blood was collected to count CLL cells, and spleens were harvested and weighed [2] 2. VEGF-dependent MM xenograft model: Nude mice were inoculated with VEGF-overexpressing MM cells subcutaneously; Lenalidomide (Revlimid, CC5013) was administered orally at 7.5 mg/kg once daily for 14 days; tumor tissues were collected, and vascular density was analyzed by CD31 immunohistochemistry [2] 1. CRBN-wildtype/knockout H929 xenograft model: Nude mice were subcutaneously inoculated with CRBN-wildtype or CRBN-knockout H929 cells (5×10⁶ cells/mouse); Lenalidomide (Revlimid, CC5013) was administered orally at 10 mg/kg once daily for 21 days; tumor growth was monitored, and tumor inhibition rates were calculated [3] 1. MV4-11 AML xenograft model: Nude mice were subcutaneously inoculated with 5×10⁶ MV4-11 cells; when tumors reached ~80 mm³, mice were treated with Lenalidomide (Revlimid, CC5013) (10 mg/kg/day) alone or in combination with CKIα/CDK7/9 inhibitors (5 mg/kg each) via oral gavage for 28 days; tumor volume was measured every 2 days, and mouse survival was recorded for 60 days [5] 1. Mouse pharmacokinetic assay: C57BL/6 mice (18–22 g) were orally administered Lenalidomide (Revlimid, CC5013) at 10 mg/kg (dissolved in 10% DMSO/40% PEG400/50% water); blood and tissue samples (liver, brain, kidney, tumor) were collected at 0.5, 1, 2, 4, 8, and 24 h post-dosing; Lenalidomide (Revlimid, CC5013) concentrations were quantified by HPLC-MS [4] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following oral administration, lenalidomide is rapidly absorbed with high bioavailability. It has a Tmax ranging from 0.5 to six hours. Lenalidomide exhibits a linear pharmacokinetic profile, with its AUC and Cmax increasing proportionally with dose. Multiple dosing does not result in drug accumulation. In healthy male subjects, the Cmax was 413 ± 77 ng/ml and the AUCinfinity was 1319 ± 162 h x ng/ml. Lenalidomide is eliminated predominantly via urinary excretion in the unchanged form. Following oral administration of 25 mg of radiolabeled lenalidomide in healthy subjects, about 90% of the dose (4.59% as metabolites) was eliminated in urine and 4% of the dose (1.83% as metabolites) was eliminated in feces within ten days post-dose. Approximately 85% of the dose was excreted as lenalidomide in the urine within 24 hours. In healthy male subjects, the apparent volume of distribution was 75.8 ± 7.3 L. The renal clearance of lenalidomide exceeds the glomerular filtration rate. In healthy male subjects, the oral clearance was 318 ± 41 mL/min. In vitro (14)C-lenalidomide binding to plasma proteins is approximately 30%. Administration of a single 25 mg dose of Revlimid with a high-fat meal in healthy subjects reduces the extent of absorption, with an approximate 20% decrease in AUC and 50% decrease in Cmax. In the trials where the efficacy and safety were established for Revlimid, the drug was administered without regard to food intake. Revlimid can be administered with or without food. Systemic exposure (AUC) of lenalidomide in multiple myeloma (MM) and myelodysplastic syndromes (MDS) patients with normal or mild renal function (CLcr = 60 mL/min) is approximately 60% higher as compared to young healthy male subjects. Lenalidomide is rapidly absorbed following oral administration. Following single and multiple doses of Revlimid in patients with multiple myeloma (MM) or myelodysplastic syndromes (MDS) the maximum plasma concentrations occurred between 0.5 and 6 hours post-dose. The single and multiple dose pharmacokinetic disposition of lenalidomide is linear with AUC and Cmax values increasing proportionally with dose. Multiple dosing at the recommended dose-regimen does not result in drug accumulation. For more Absorption, Distribution and Excretion (Complete) data for Lenalidomide (9 total), please visit the HSDB record page. Metabolism / Metabolites Lenalidomide is not subject to extensive hepatic metabolism involving CYP enzymes and metabolism contributes to a very minor extent to the clearance of lenalidomide in humans. Lenalidomide undergoes hydrolysis in human plasma to form 5-hydroxy-lenalidomide and N-acetyl-lenalidomide. Unchanged lenalidomide is the predominant circulating drug form, with metabolites accounting for less than five percent of the parent drug levels in the circulation. Lenalidomide -undergoes limited metabolism. Unchanged lenalidomide is the predominant circulating component in humans. Two identified metabolites are hydroxy-lenalidomide and N-acetyl-lenalidomide; each constitutes less than 5% of parent levels in circulation. Biological Half-Life In healthy subjects, the mean half-life of lenalidomide is three hours in the clinically relevant dose range (5–50 mg). Half-life can range from three to five hours in patients with multiple myeloma, myelodysplastic syndromes, or mantle cell lymphoma. The mean half-life of lenalidomide is 3 hours in healthy subjects and 3 to 5 hours in patients with multiple myeloma (MM), myelodysplastic syndromes (MDS) or mantle cell lymphoma (MCL). 1. Absorption: In C57BL/6 mice, oral administration of Lenalidomide (Revlimid, CC5013) (10 mg/kg) reached a peak plasma concentration (Cmax) of 3.2 μM at 1 h post-dosing, with an oral bioavailability of ~90% [4] 2. Distribution: Lenalidomide (Revlimid, CC5013) was widely distributed in mouse tissues, with the highest concentration in the liver (Cmax = 5.1 μM), followed by the kidney (Cmax = 3.8 μM), and the lowest in the brain (Cmax = 0.3 μM); the tumor/plasma concentration ratio was 1.2 [4] 3. Metabolism: Lenalidomide (Revlimid, CC5013) was primarily metabolized in the mouse liver via hydrolysis to form inactive lenalidomide acid; it was not metabolized by cytochrome P450 enzymes [4] 4. Excretion: In mice, ~60% of the administered dose of Lenalidomide (Revlimid, CC5013) was excreted in feces within 72 h, and ~30% in urine; unchanged drug accounted for ~40% of the excreted dose [4] 5. Half-life: The elimination half-life (t1/2) of Lenalidomide (Revlimid, CC5013) in mouse plasma was 4.5 h [4] |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Lenalidomide is off-white to pale-yellow solid powder. Lenalidomide, a thalidomide analog, is an immunomodulatory agent with antineoplastic and antiangiogenic activity. Lenalidomide is used for the treatment of patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes (MDS) associated with a deletion 5q cytogenetic abnormality with or without additional cytogenetic abnormalities. It is also used for the treatment of patients with mantle cell lymphoma (MCL) whose disease has relapsed or progressed after two prior therapies, one of which included bortezomib. It is used in combination with dexamethasone for the treatment of patients with multiple myeloma (MM) who have received at least one prior therapy. HUMAN EXPOSURE AND TOXICITY: Lenalidomide can cause fetal harm when administered to a pregnant female. Limb abnormalities were seen in the offspring of monkeys that were dosed with lenalidomide during organogenesis. This effect was seen at all doses tested. Due to the results of this developmental monkey study, and lenalidomide's structural similarities to thalidomide, a known human teratogen, lenalidomide is contraindicated in females who are pregnant. Lenalidomide has demonstrated a significantly increased risk of deep vein thrombosis (DVT) and pulmonary embolism (PE), as well as risk of myocardial infarction and stroke in patients with multiple myeloma who were treated with lenalidomide and dexamethasone therapy. Hepatic failure, including fatal cases, has occurred in patients treated with lenalidomide in combination with dexamethasone. Patients with multiple myeloma treated with lenalidomide in studies including melphalan and stem cell transplantation had a higher incidence of second primary malignancies, particularly acute myelogenous leukemia (AML) and Hodgkin lymphoma, compared to patients in the control arms who received similar therapy but did not receive lenalidomide. Lenalidomide can cause significant neutropenia and thrombocytopenia. Fatal instances of tumor lysis syndrome have been reported during treatment with lenalidomide. The patients at risk of tumor lysis syndrome are those with high tumor burden prior to treatment. Angioedema and serious dermatologic reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis, have been reported in lenalidomide-treated patients. These reactions can be fatal. ANIMAL STUDIES: Acute administration of lenalidomide to rats and mice via intravenous and oral administration did not result in mortality. However, repeated oral administration of 4 and 6 mg/kg/day to monkeys for up to 20 weeks produced mortality and significant toxicity. The embryotoxic and teratogenic potential of lenalidomide was studied in rats, rabbits and monkeys. A pre- and post-natal development study in rats revealed few adverse effects in the offspring of female rats treated with lenalidomide at doses up to 500 mg/kg. In rabbits, no limb abnormalities were attributable to lenalidomide in the offspring of females administered 3, 10 and 20 mg/kg/day orally. Developmental toxicity at the 10 and 20 mg/kg/day dose levels was characterized by slightly reduced fetal body weights, increased incidences of post implantation loss (early and late resorptions and intrauterine deaths), and gross external findings in the fetuses associated with morbidity and pharmacotoxic effects of lenalidomide (purple discoloration of the skin on the entire body). An absence of the intermediate lobe of the lung was observed at 10 and 20 mg/kg/day with dose dependence and displaced kidneys were observed at 20 mg/kg/day. Soft tissue and skeletal variations in the fetuses were also observed at 10 and 20 mg/kg/day. These included minor variations in skull ossification (irregular nasal-frontal suture) and small delays in ossification of the metacarpals, associated with the reduced fetal body weights. In monkeys, malformations were observed in the offspring of female monkeys who received lenalidomide doses as low as 0.5 mg/kg/day during pregnancy. The observed malformations ranged from stiff and slightly malrotated hindlimbs at 0.5 mg/kg/day of lenalidomide up to severe external malformations, such as bent, shortened, malformed, malrotated, and/or absent part of the extremities, oligo- or polydactyly at 4 mg/kg/day of lenalidomide. Lenalidomide was not mutagenic in the bacterial reverse mutation assay (Ames test) and did not induce chromosome aberrations in cultured human peripheral blood lymphocytes, or mutations at the thymidine kinase (tk) locus of mouse lymphoma L5178Y cells. Lenalidomide did not increase morphological transformation in Syrian Hamster Embryo assay or induce micronuclei in the polychromatic erythrocytes of the bone marrow of male rats. Hepatotoxicity Serum enzyme elevations occur in 8% to 15% of patients taking lenalidomide and are more frequent with higher doses. The enzyme abnormalities are usually mild and self-limited, and only rarely require drug discontinuation. In addition, lenalidomide has been implicated in rare instances of clinically apparent, acute liver injury which can be severe and has led to deaths from acute liver failure. The onset of injury is typically within 1 to 8 weeks of starting therapy. The pattern of serum enzyme elevation at the time of presentation can be either hepatocellular or cholestatic; however, the injury tends to be cholestatic and can be prolonged. Immunoallergic and autoimmune features are not common. Several instances of acute liver injury associated with lenalidomide therapy have occurred in patients with other apparent causes of liver disease or with preexisting chronic hepatitis B or C. If performed during the acute injury, liver biopsy shows hepatocellular necrosis and inflammatory cell infiltration, consistent with acute drug induced injury. In some instances there is bile duct injury and loss resulting in progressive cholestatic liver injury suggestive of vanishing bile duct syndrome. Lenalidomide has also been shown to increase indirect bilirubin levels in patients with underlying Gilbert syndrome, causing a mild hyperbilirubinemia during therapy that soon resolves with stopping treatment and is otherwise benign. Thalidomide and its derivatives have also been implicated in causing an increased risk of graft-vs-host disease after autologous or allogeneic hematopoietic stem cell transplantation (HSCT) as well as after liver, kidney and heart transplantation. There appears to be cross reactivity to this complication among lenalidomide, pomalidomide and thalidomide. Therapy usually requires discontinuation of the antineoplastic agent as well as treatment with high doses of corticosteroids and tacrolimus or sirolimus. Furthermore, hepatic graft-vs-host disease can occasionally present with an acute hepatitis that resembles hepatocellular drug induced liver injury. Reactivation of hepatitis B has been reported in patients receiving thalidomide, lenalidomide and pomalidomide, but generally only after HSCT and the role of these agents in causing reactivation is not always clear. Indeed, in studies of large numbers of patients treated for multiple myeloma, the major risk factor for reactivation was found to be HSCT rather than the specific antineoplastic drugs being used. Indeed, lenalidomide therapy is associated with a reduced risk of reactivation in patients with HSCT (although dexamethasone, thalidomide and bortezomib were not), perhaps because of the immune enhancement typically caused by lenalidomide. Likelihood score: C (probable rare cause of clinically apparent liver injury). Protein Binding _In vitro_, about 30% of lenalidomide was bound to plasma proteins. Interactions Erythropoietic agents, or other agents that may increase the risk of thrombosis, such as estrogen containing therapies, should be used with caution after making a benefit-risk assessment in patients receiving Revlimid. Co-administration of multiple dose Revlimid (10 mg) with single dose warfarin (25 mg) had no effect on the pharmacokinetics of total lenalidomide or R- and S-warfarin. Expected changes in laboratory assessments of PT and INR were observed after warfarin administration, but these changes were not affected by concomitant Revlimid administration. It is not known whether there is an interaction between dexamethasone and warfarin. Close monitoring of PT and INR is recommended in multiple myeloma patients taking concomitant warfarin. When digoxin was co-administered with multiple doses of Revlimid (10 mg/day) the digoxin Cmax and AUC0-8 were increased by 14%. Periodic monitoring of digoxin plasma levels, in accordance with clinical judgment and based on standard clinical practice in patients receiving this medication, is recommended during administration of Revlimid. 1. In vitro cytotoxicity: Lenalidomide (Revlimid, CC5013) (50 μM) showed no cytotoxicity to normal human PBMCs, with cell viability >90% (MTT assay) [2] 2. In vivo toxicity: Mice treated with Lenalidomide (Revlimid, CC5013) (50 mg/kg/day, oral for 28 days) had no significant changes in body weight, food intake, or serum biochemical parameters (ALT, AST, BUN, Cr) [2] 1. Acute toxicity: The oral LD50 of Lenalidomide (Revlimid, CC5013) in mice was >2000 mg/kg [4] 2. Subchronic toxicity: Rats treated with Lenalidomide (Revlimid, CC5013) (100 mg/kg/day, oral for 90 days) showed no histopathological abnormalities in the liver, kidney, heart, or spleen; serum biochemical indicators were within the normal range [4] 3. Plasma protein binding: The plasma protein binding rate of Lenalidomide (Revlimid, CC5013) in mouse plasma was ~30% (ultrafiltration method) [4] 1. Combination toxicity: Mice treated with Lenalidomide (Revlimid, CC5013) combined with CKIα/CDK7/9 inhibitors showed mild weight loss (<5%) and a 20% increase in serum ALT, which reversed after drug withdrawal [5] 1. In vitro cytotoxicity: Lenalidomide (Revlimid, CC5013) (50 μM) had no cytotoxicity to primary astrocytes, with cell viability >95% (MTT assay) [7] |
| 参考文献 |
[1]. Krönke J, et al. Lenalidomide induces degradation of IKZF1 and IKZF3. Oncoimmunology. 2014 Jul 3;3(7):e941742.
[2]. Kotla V, et al. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol. 2009 Aug 12;2:36. [3]. Lopez-Girona A, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012 Nov;26(11):2326-35. [4]. Rozewski DM, et al. Pharmacokinetics and tissue disposition of lenalidomide in mice. AAPS J. 2012 Dec;14(4):872-82. [5]. Minzel W, et al. Small Molecules Co-targeting CKIα and the Transcriptional Kinases CDK7/9 Control AML in Preclinical Models. Cell. 2018 Sep 20;175(1):171-185.e25. [6]. Nagashima, Takeyuki, et al. PHARMACEUTICAL COMPOSITION COMPRISING BICYCLIC NITROGEN-CONTAINING AROMATIC HETEROCYCLIC AMIDE COMPOUND AS ACTIVE INGREDIENT. Patent. 20170360780A1. [7]. Omran A, et al. Effects of MRP8, LPS, and lenalidomide on the expressions of TNF-α , brain-enriched, and inflammation-related microRNAs in the primary astrocyte culture. ScientificWorldJournal. 2013 Sep 21;2013:20830 |
| 其他信息 |
Therapeutic Uses
Angiogenesis Inhibitors; Immunologic Factors Revlimid in combination with dexamethasone is indicated for the treatment of patients with multiple myeloma (MM) who have received at least one prior therapy. /Included in US product label/ Revlimid is indicated for the treatment of patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes (MDS) associated with a deletion 5q cytogenetic abnormality with or without additional cytogenetic abnormalities. /Included in US product label/ Revlimid is indicated for the treatment of patients with mantle cell lymphoma (MCL) whose disease has relapsed or progressed after two prior therapies, one of which included bortezomib. /Included in US product label/ For more Therapeutic Uses (Complete) data for Lenalidomide (7 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ WARNING: EMBRYO-FETAL TOXICITY. Do not use Revlimid during pregnancy. Lenalidomide, a thalidomide analogue, caused limb abnormalities in a developmental monkey study. Thalidomide is a known human teratogen that causes severe life-threatening human birth defects. If lenalidomide is used during pregnancy, it may cause birth defects or embryo-fetal death. In females of reproductive potential, obtain 2 negative pregnancy tests before starting Revlimid treatment. Females of reproductive potential must use 2 forms of contraception or continuously abstain from heterosexual sex during and for 4 weeks after Revlimid treatment. To avoid embryo-fetal exposure to lenalidomide, Revlimid is only available through a restricted distribution program, the Revlimid REMS program (formerly known as the RevAssist program). /BOXED WARNING/ WARNING: HEMATOLOGIC TOXICITY. Revlimid can cause significant neutropenia and thrombocytopenia. Eighty percent of patients with del 5q myelodysplastic syndromes had to have a dose delay/reduction during the major study. Thirty-four percent of patients had to have a second dose delay/reduction. Grade 3 or 4 hematologic toxicity was seen in 80% of patients enrolled in the study. Patients on therapy for del 5q myelodysplastic syndromes should have their complete blood counts monitored weekly for the first 8 weeks of therapy and at least monthly thereafter. Patients may require dose interruption and/or reduction. Patients may require use of blood product support and/or growth factors /BOXED WARNING/ WARNING: VENOUS AND ARTERIAL THROMBOEMBOLISM. Revlimid has demonstrated a significantly increased risk of deep vein thrombosis (DVT) and pulmonary embolism (PE), as well as risk of myocardial infarction and stroke in patients with multiple myeloma who were treated with Revlimid and dexamethasone therapy. Monitor for and advise patients about signs and symptoms of thromboembolism. Advise patients to seek immediate medical care if they develop symptoms such as shortness of breath, chest pain, or arm or leg swelling. Thromboprophylaxis is recommended and the choice of regimen should be based on an assessment of the patient's underlying risks. Patients with multiple myeloma treated with lenalidomide in studies including melphalan and stem cell transplantation had a higher incidence of second primary malignancies, particularly acute myelogenous leukemia (AML) and Hodgkin lymphoma, compared to patients in the control arms who received similar therapy but did not receive lenalidomide. Monitor patients for the development of second malignancies. Take into account both the potential benefit of lenalidomide and the risk of second primary malignancies when considering treatment with lenalidomide. For more Drug Warnings (Complete) data for Lenalidomide (19 total), please visit the HSDB record page. Pharmacodynamics In hematological malignancies, the immune system is deregulated in the form of altered cytokine networks in the tumour microenvironment, defective T cell regulation of host-tumour immune interactions, and diminished NK cell activity. Lenalidomide is an immunomodulatory agent with antineoplastic, antiangiogenic, and anti-inflammatory properties. Lenalidomide exerts direct cytotoxicity by increasing apoptosis and inhibiting the proliferation of hematopoietic malignant cells. It delays tumour growth in nonclinical hematopoietic tumour models _in vivo_, including multiple myeloma. Lenalidomide also works to limit the invasion or metastasis of tumour cells and inhibits angiogenesis. Lenalidomide also mediates indirect antitumour effects via its immunomodulatory actions: it inhibits the production of pro-inflammatory cytokines, which are implicated in various hematologic malignancies. Lenalidomide enhances the host immunity by stimulating T cell proliferation and enhancing the activity of natural killer (NK) cells. Lenalidomide is about 100–1000 times more potent in stimulating T cell proliferation than [thalidomide]. _In vitro_, it enhances antibody-dependent cell-mediated cytotoxicity (ADCC), which is even more pronounced when used in combination with rituximab. Due to its anti-inflammatory properties, lenalidomide has been investigated in the context of inflammatory and autoimmune diseases, such as amyotrophic lateral sclerosis. 1. Lenalidomide (Revlimid, CC5013) is an immunomodulatory imide drug (IMiD) and a structural analog of thalidomide; its anti-myeloma activity is mediated by CRBN-dependent degradation of IKZF1 and IKZF3, two transcription factors critical for MM cell survival [1] 1. Lenalidomide (Revlimid, CC5013) is approved by the FDA for the treatment of multiple myeloma (in combination with dexamethasone), myelodysplastic syndromes (MDS) with del(5q) cytogenetic abnormality, and mantle cell lymphoma (MCL) [2] 2. Its mechanisms of action include immunomodulation (enhancing T/NK cell activity), anti-angiogenesis (inhibiting VEGF/ bFGF), and direct anti-tumor effects (inducing apoptosis in malignant B cells) [2] 1. CRBN is an essential target for Lenalidomide (Revlimid, CC5013); CRBN mutations or downregulation lead to acquired resistance to Lenalidomide (Revlimid, CC5013) in MM patients [3] 1. Combination of Lenalidomide (Revlimid, CC5013) with CKIα and CDK7/9 inhibitors is a promising therapeutic strategy for AML, overcoming the limited single-agent efficacy of Lenalidomide (Revlimid, CC5013) in AML [5] 1. Lenalidomide (Revlimid, CC5013) modulates microRNA expression and TNF-α production in astrocytes, suggesting potential applications in the adjuvant treatment of neuroinflammatory diseases [7] |
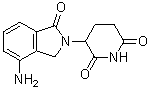
| 分子式 |
C13H13N3O3
|
|---|---|
| 分子量 |
259.2606
|
| 精确质量 |
259.095
|
| 元素分析 |
C, 60.22; H, 5.05; N, 16.21; O, 18.51
|
| CAS号 |
191732-72-6
|
| 相关CAS号 |
Lenalidomide hemihydrate;847871-99-2;Lenalidomide hydrochloride;1243329-97-6;Lenalidomide sodium;Lenalidomide-d5;1227162-34-6
|
| PubChem CID |
216326
|
| 外观&性状 |
Off-white to light yellow solid
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
614.0±55.0 °C at 760 mmHg
|
| 熔点 |
269-271°C
|
| 闪点 |
325.1±31.5 °C
|
| 蒸汽压 |
0.0±1.8 mmHg at 25°C
|
| 折射率 |
1.672
|
| LogP |
-1.39
|
| tPSA |
92.5
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
19
|
| 分子复杂度/Complexity |
437
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C1C2C([H])=C([H])C([H])=C(C=2C([H])([H])N1C1([H])C(N([H])C(C([H])([H])C1([H])[H])=O)=O)N([H])[H]
|
| InChi Key |
GOTYRUGSSMKFNF-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C13H13N3O3/c14-9-3-1-2-7-8(9)6-16(13(7)19)10-4-5-11(17)15-12(10)18/h1-3,10H,4-6,14H2,(H,15,17,18)
|
| 化学名 |
3-(4-amino-1-oxoisoindolin-2-yl)piperidine-2,6-dione
|
| 别名 |
CC5013; CC-5013; CC 5013; IMiD1; Lenalidomide; Brand name: Revlimid.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~385.71 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.64 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.64 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (9.64 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.5 mg/mL (9.6 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + + 45% Saline ≥ 2.5 mg/mL (9.6 mM) in 10% DMSO + 90% (20% SBE-β-CD in saline) ≥ 2.5 mg/mL (9.6 mM) in 10% DMSO + 90% Corn oil 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.8571 mL | 19.2857 mL | 38.5713 mL | |
| 5 mM | 0.7714 mL | 3.8571 mL | 7.7143 mL | |
| 10 mM | 0.3857 mL | 1.9286 mL | 3.8571 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Study of VAY736 as Single Agent and in Combination With Select Antineoplastic Agents in Patients With Non-Hodgkin Lymphoma
CTID: NCT04903197
Phase: Phase 1 Status: Active, not recruiting
Date: 2024-11-29