| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
| 靶点 |
CysLT1/cysteinyl leukotriene receptor 1
|
|---|---|
| 体外研究 (In Vitro) |
孟鲁司特(5 μM;1 小时)可防止对乙酰氨基酚 (APAP) 引起的细胞损伤 [1]。孟鲁司特 (0.01-10 μM) 给药 30 分钟可抑制 5-oxo-ETE 产生的细胞迁移,并改变纤溶酶-纤溶酶原系统的激活 [3]。 10 μM 孟鲁司特持续 18 小时会改变 MMP-9 活性 [3]。
|
| 体内研究 (In Vivo) |
孟鲁司特(3 mg/kg;口服)可保护小鼠免受 APAP 引起的肝毒性 [1]。当通过微渗透泵给药时,孟鲁司特 (1 mg/kg) 通过 CysLT1 受体抑制半胱氨酰白三烯 (LT) 的产生,并减轻 OVA 治疗小鼠气道重塑的改变。 C4、D4和E4的角色[2]。使用微渗透泵给予 1 mg/kg 孟鲁司特,可以降低用 OVA 治疗的小鼠 BAL 液中 IL-4 和 IL-13 水平的升高 [2]。
|
| 酶活实验 |
孟鲁司特和MK-0591降低了5-氧代-ETE促进的嗜酸性粒细胞迁移,而LTD(4)未能诱导嗜酸性粒细胞核迁移。然而,LTD(4)显著提高了用次优浓度的5-氧代-ETE获得的迁移速率,并部分逆转了用MK-0591获得的抑制作用。孟鲁司特显著降低了用5-氧代-ETE获得的嗜酸性粒细胞将纤溶酶原活化为纤溶酶的最大速率。5-Oxo-ETE增加了表达尿激酶纤溶酶原激活物受体的嗜酸性粒细胞的数量,并刺激了MMP-9的分泌。孟鲁司特,但MK-0591和LTD(4)均未降低尿激酶纤溶酶原激活剂受体的表达和MMP-9的分泌,并增加尿激酶纤溶酶原活化剂的总细胞活性和纤溶酶原激活物抑制剂2mRNA的表达[3]。
|
| 细胞实验 |
细胞迁移测定 [3]
细胞类型: 嗜酸性粒细胞 测试浓度: 0.01-10 μM 孵育时间: 30 分钟 实验结果:减少 5-oxo-ETE 诱导的细胞迁移。 蛋白质印迹分析[3] 细胞类型: 嗜酸性粒细胞 测试浓度: 10 μM 孵育时间: 18 小时 实验结果: 5-oxo-ETE 促进的 MMP-9 分泌减少。 |
| 动物实验 |
Animal/Disease Models: C57BL/6J mice (8 weeks old; 22-25 g) induced acute liver injury [1]
Doses: 3 mg/kg Route of Administration: po (oral gavage) 1 hour after administration of normal saline or APAP Experimental Results: Serum moderate alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and reduce liver damage. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
It has been observed that montelukast is quickly absorbed following administration by the oral route. The oral bioavailability documented for the drug is 64%. Furthermore, it seems that having a regular meal in the morning or even a high fat snack in the evening does not affect the absorption of montelukast. It has been reported that montelukast and its metabolites are almost exclusively excreted in the bile and into the feces. The steady-state volume of distribution recorded for montelukast is an average between 8 to 11 litres. The plasma clearance documented for montelukast is an average of 45 mL/min when observed in healthy adults. Montelukast is rapidly absorbed from the GI tract, and peak plasma concentrations are attained within 3-4, 2-2.5, or 2 hours following oral administration in the fasted state of a single 10-mg film-coated (in adults), 5-mg chewable (in adults), or 4-mg chewable (in children 2-5 years of age) tablet, respectively. ... Ingestion of a high-fat meal in the morning with the 4-mg oral granules formulation had no effect on the AUC of montelukast; however, the time to peak plasma concentrations was prolonged from 2.3 hours to 6.4 hours and peak plasma concentrations were reduced by 35%. Absorption /of montelukast is/ rapid. For the 10-mg tablets: mean oral bioavailability is 64%. Bioavailability is not affected by a standard meal in the morning. For the 5-mg chewable tablet: mean oral bioavailability is 73% in the fasted state versus 63% when administered with a standard meal in the morning. Following oral administration of montelukast 10 mg daily for 7 days in fasting young adults, peak plasma concentrations averaged 541 ng/mL on day 1 and 602.8 ng/mL on day 7. Trough concentrations on days 3-7 were essentially constant and ranged from 18-24 ng/mL. In this study, values for area under the plasma concentration-time curve (AUC) at steady-state were about 14-15% higher than those achieved with a single dose, and were reached within 2 days. The pharmacokinetics of montelukast are nearly linear at doses of up to 50 mg. For more Absorption, Distribution and Excretion (Complete) data for MONTELUKAST (15 total), please visit the HSDB record page. Metabolism / Metabolites It has been determined that montelukast is highly metabolized and typically so by the cytochrome P450 3A4, 2C8, and 2C9 isoenzymes. In particular, it seems that the CYP2C8 enzymes play a significant role in the metabolism of the drug. Nevertheless, at therapeutic doses, the plasma concentrations of montelukast metabolites are undetectable at steady state in adults and pediatric patients. Biotransformation /is/ hepatic and extensive involving cytochrome P450 3A4 and 2C9 The metabolic fate of montelukast has not been fully determined, but the drug is extensively metabolized in the GI tract and/or liver and excreted in bile. Several metabolic pathways have been identified including acyl glucuronidation, and oxidation catalyzed by several cytochrome P-450 (CYP) isoenzymes. In vitro studies indicate that the microsomal P-450 isoenzyme CYP3A4 is the major enzyme involved in formation of the 21-hydroxy metabolite (M5) and a sulfoxide metabolite (M2), and CYP2C9 is the major isoenzyme involved in the formation of the 36-hydroxy metabolite (M6). Other identified metabolites include an acyl glucuronide (M1) and a 25-hydroxy (a phenol, M3) analog. Following oral administration of 54.8 mg of radiolabeled montelukast, metabolites of the drug represented less than 2% of circulating radioactivity. Montelukast metabolites that have been identified in plasma in radiolabeled studies include the 21-hydroxy (diastereomers of a benzylic acid, M5a and M5b) and the 36-hydroxy (diastereomers of a methyl alcohol, M6a and M6b) metabolites. Following oral administration of therapeutic doses of montelukast, plasma concentrations of metabolites at steady-state in adults and children were below the level of detection. Montelukast has known human metabolites that include montelukast sulfoxide, Montelukast 1, 2-Diol, 21-Hydroxymontelukast, and 21(S)-Hydroxy Montelukast. Biological Half-Life Studies have demonstrated that the mean plasma half-life of montelukast varies from 2.7 to 5.5 hours when observed in healthy young adults. The mean plasma elimination half-life of montelukast in adults 19-48 years of age is 2.7-5.5 hours, and plasma clearance averages 45 mL/minute. A plasma elimination half-life of 3.4-4.2 hours has been reported in children 6-14 years of age. Limited data indicate that the plasma elimination half-life of montelukast is prolonged slightly in geriatric adults and in patients with mild to moderate hepatic impairment, although dosage adjustment is not required. A plasma elimination half-life of 6.6 or 7.4 hours has been reported in geriatric adults 65-73 years of age or patients with mild to moderate hepatic impairment, respectively. |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
Concurrent use /of phenobarbital/ results in significant decreases (approximately 40%) in the area under the curve [AUC] for montelukast, of induction of hepatic metabolism... ... This study was designed to evaluate whether montelukast at clinically used dosage levels would interfere with the anticoagulant effect of warfarin. In a two-period, double-blind, randomized crossover study, 12 healthy male subjects received a single oral dose of 30 mg warfarin on the 7th day of a 12-day treatment with montelukast, 10 mg daily by mouth, or a placebo. Montelukast had no significant effect on the area under the plasma concentration-time curves and peak plasma concentrations of either R- or S-warfarin. However, slight but statistically significant decreases in time to peak concentration of both warfarin enantiomers and in elimination half-life of the less potent R-warfarin were observed in the presence of montelukast. These changes were not considered as clinically relevant. Montelukast had no significant effect on the anticoagulant effect of warfarin, as assessed by the international normalized ratio (INR) for prothrombin time (AUC0-144 and INR maximum). The results of this study suggest that a clinically important interaction between these drugs is unlikely to occur in patients requiring concomitant administration of both drugs. The effect of montelukast (MK-0476), a cysteinyl leukotriene receptor antagonist, ... on single-dose theophylline plasma concentrations was studied in three separate clinical trials. Montelukast was evaluated at 10 mg once daily (the clinical dosage), 200 mg once daily, and 600 mg (200 mg three times daily). At the clinical dosage, montelukast did not change single-dose theophylline plasma concentration in a clinically important manner. The geometric mean ratios for theophylline area under the plasma concentration versus time curve (AUC0-->infinity ) (0.92) and maximal plasma concentration (Cmax ) (1.04) were well within the predefined and generally accepted bioequivalence range of 0.80 and 1.25. Montelukast decreased theophylline Cmax by 12% and 10%, AUC0-->infinity by 43% and 44%, and elimination half-time by 44% and 39% at 200 mg/d (oral and intravenous, respectively), and at 600 mg/d, montelukast decreased theophylline Cmax by 25%, AUC0-->infinity by 66%, and elimination half-time by 63%. These results show that montelukast at the clinical dosage did not change theophylline pharmacokinetics in a clinically important manner, but at 20- to 60-fold higher dosages, montelukast significantly reduced the theophylline pharmacokinetics parameters; an apparent dosage dependence is suggested. High aminotransferases and prolonged prothrombin time on entering our liver unit were revealing parenchymal collapse for this 45-year-old obese woman; treatment failure led her to death. Autoimmunity, paracetamol use, alcoholism, and Wilson's disease were all excluded as causes. Because of chronic asthma, she had been receiving a leukotriene receptor antagonist (montelukast) for 5 years before the current presentation; 1 week before onset she had had 1 week of treatment with two dietary supplements for weight control; one of these included Garcinia Cambogia, a possible cause of two recent cases of hepatitis in the USA; in addition, both formulas contained a citrus derivative that interferes cytochrome functions. /The authors/ speculate on a causal relationship between the assumption of the additives and the fatal hepatitis and envisage a synergy between the additives and montelukast, which per se has well been studied as a hepatotoxic drug. Despite the speculative nature of this presentation, /investigators/ believe the warning may serve to focus attention on the uncontrolled escalation of food additives going on /at present/. ... The present case describes an asthmatic patient, who developed severe obstructive symptoms and progressive heart failure after two sequential exposures to montelukast. As the patient exhibited a markedly raised blood eosinophil count with diffuse infiltrates on chest x-ray and signs of myocarditis, Churg-Strauss syndrome (CSS) was suspected. The disease was confirmed by open lung biopsy. The symptoms improved rapidly after administration of high dose immunosuppression with methylprednisolone and cyclophosphamide. This case is noteworthy because the time course of events strongly suggests a direct aetiological role for montelukast in the development of CSS. The pathophysiological mechanism of the association remains unknown. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Anti-Asthmatic agents; Leukotriene Antagonists Montelukast is indicated for prophylaxis and chronic treatment of asthma in adults and pediatric patients 12 months of age and older. /Included in US product label/ Montelukast is indicated for the relief of symptoms of seasonal allergic rhinitis in adults and pediatric patients 2 years of age and older. /Included in US product label/ Montelukast is not indicated for treatment of bronchospasm in acute asthma attacks, including status asthmaticus. /Not included in US product label/ Drug Warnings Headache is the most frequently reported adverse effect with montelukast, occurring in 18-19% of children 6 years of age or older, adolescents, and adults. Headache has been reported in at least 2% of children 2-8 years of age with asthma receiving montelukast and in at least 1% (and more frequently than with placebo) of adults and adolescents 15 years of age or older with asthma. Sinus headache has been reported in at least 1% of adult and adolescent patients 15 years of age or older with perennial allergic rhinitis receiving montelukast and more frequently than in those receiving placebo. Dizziness or asthenia/fatigue has occurred in about 1.8-1.9% of patients 15 years of age or older receiving the drug in clinical studies. Dream abnormalities, hallucinations, agitation including aggressive behavior, paresthesia/hypoesthesia, drowsiness, insomnia, irritability, or restlessness also has been reported; seizures have been reported very rarely. Abdominal pain has occurred in 2.9% of patients 15 years of age or older receiving montelukast. Dyspepsia, infectious gastroenteritis, and dental pain have been reported in 2.1, 1.5, and 1.7% of patients in this age group, respectively. Diarrhea or nausea has been reported in at least 2% of children 6-14 years of age receiving montelukast. Abdominal pain, diarrhea, and gastroenteritis has been reported in at least 2% of children 2-5 years of age with asthma and more frequently than in those receiving placebo. Gastroenteritis has been reported in at least 2% of children 6-8 years of age with asthma and more frequently than in those receiving placebo. Nausea, vomiting, dyspepsia, pancreatitis (rarely), and diarrhea also have been reported with montelukast therapy during postmarketing experience. Elevations in the results of one or more liver function tests have occurred in patients receiving montelukast in clinical studies. Increases in serum ALT (SGPT) or AST (SGOT) concentrations occurred in 2.1 or 1.6%, respectively, of patients 15 years of age or older with asthma receiving montelukast in clinical studies. Increases in ALT occurred in at least 1% of adult and adolescent patients 15 years of age or older with perennial allergic rhinitis receiving montelukast in clinical studies and more frequently than in those receiving placebo. Changes in laboratory values returned to normal despite continuing montelukast therapy or were not directly attributable to drug therapy. Elevations in serum aminotransferase (transaminase) concentrations also have been reported in children 2-14 years of age receiving montelukast, but the incidence of these elevations was similar to that in children receiving placebo. Hepatic eosinophilic infiltration has been reported very rarely through postmarketing experience with montelukast. Hepatocellular injury, cholestatic hepatitis, or mixed-pattern liver injury also has been reported rarely through postmarketing experience with montelukast. Confounding factors were present in most of these patients, such as the concomitant use of other drugs or alcohol or in the presence of coexisting conditions (e.g., other forms of hepatitis). Rash has occurred in 1.6% of adults and adolescents 15 years of age or older receiving montelukast. Rash, eczema, dermatitis, or urticaria has been reported in at least 2% of children 2-5 years of age receiving the drug. Atopic dermatitis, varicella, and skin infection have been reported in at least 2% of children 6-8 years of age with asthma receiving montelukast and more frequently than in those receiving placebo. Hypersensitivity reactions, including anaphylaxis, angioedema, pruritus, urticaria, and rarely hepatic eosinophilic infiltration, have been reported in patients receiving montelukast. For more Drug Warnings (Complete) data for MONTELUKAST (17 total), please visit the HSDB record page. Pharmacodynamics Montelukast is a leukotriene receptor antagonist that demonstrates a marked affinity and selectivity to the cysteinyl leukotriene receptor type-1 in preference to many other crucial airway receptors like the prostanoid, cholinergic, or beta-adrenergic receptors. As a consequence, the agent can elicit substantial blockage of LTD4 leukotriene-mediated bronchoconstriction with doses as low as 5 mg. Moreover, a placebo-controlled, crossover study (n=12) demonstrated that montelukast is capable of inhibiting early and late phase bronchoconstriction caused by antigen challenge by 75% and 57% respectively. In particular, it has been documented that montelukast can cause bronchodilation as soon as within 2 hours of oral administration. This action can also be additive to the bronchodilation caused by the concomitant use of a beta agonist. Nevertheless, clinical investigations performed with adults 15 years of age and older revealed that no additional clinical benefit is obtained when doses of montelukast greater than 10 mg a day are used. Additionally, in clinical trials with adults and pediatric asthmatic patients aged 6 to 14 years, it was also determined that montelukast can reduce mean peripheral blood eosinophils by about 13% to 15% from baseline in comparison to placebo during double-blind treatment periods. At the same time, in patients aged 15 years and older who were experiencing seasonal allergic rhinitis, the use of montelukast caused a median reduction of 13% in peripheral blood eosinophil counts when compared to placebo as well. |
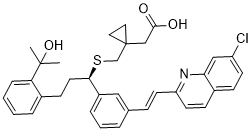
| 分子式 |
C35H36CLNO3S
|
|---|---|
| 分子量 |
586.18
|
| 精确质量 |
585.21
|
| 元素分析 |
C, 71.72; H, 6.19; Cl, 6.05; N, 2.39; O, 8.19; S, 5.47
|
| CAS号 |
158966-92-8
|
| 相关CAS号 |
Montelukast sodium;151767-02-1;Montelukast dicyclohexylamine;577953-88-9;Montelukast-d6;1093746-29-2
|
| PubChem CID |
5281040
|
| 外观&性状 |
Light yellow to yellow solid
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
750.5±60.0 °C at 760 mmHg
|
| 闪点 |
407.7±32.9 °C
|
| 蒸汽压 |
0.0±2.6 mmHg at 25°C
|
| 折射率 |
1.678
|
| LogP |
7.8
|
| tPSA |
95.72
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
12
|
| 重原子数目 |
41
|
| 分子复杂度/Complexity |
891
|
| 定义原子立体中心数目 |
1
|
| SMILES |
CC(C)(C1=CC=CC=C1CC[C@H](C2=CC=CC(=C2)/C=C/C3=NC4=C(C=CC(=C4)Cl)C=C3)SCC5(CC5)CC(=O)O)O
|
| InChi Key |
UCHDWCPVSPXUMX-TZIWLTJVSA-N
|
| InChi Code |
InChI=1S/C35H36ClNO3S/c1-34(2,40)30-9-4-3-7-25(30)13-17-32(41-23-35(18-19-35)22-33(38)39)27-8-5-6-24(20-27)10-15-29-16-12-26-11-14-28(36)21-31(26)37-29/h3-12,14-16,20-21,32,40H,13,17-19,22-23H2,1-2H3,(H,38,39)/b15-10+/t32-/m1/s1
|
| 化学名 |
Cyclopropaneacetic acid, 1-((((1R)-1-(3-((1E)-2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3-(2-(1-hydroxy-1-methylethyl)phenyl)propyl)thio)methyl)-
|
| 别名 |
MK-476; MK 476; MK0476; Brondilat; Aerokast; 142522-28-9; UNII-MHM278SD3E; MHM278SD3E; trade names Singulair; Monteflo; Lukotas; Lumona
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~250 mg/mL (~426.49 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.08 mg/mL (3.55 mM) in 10% DMSO + 40% PEG300 +5% Tween-80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80+,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7060 mL | 8.5298 mL | 17.0596 mL | |
| 5 mM | 0.3412 mL | 1.7060 mL | 3.4119 mL | |
| 10 mM | 0.1706 mL | 0.8530 mL | 1.7060 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Clinical Evaluation of Montelukast in Veterans with Gulf War Illness
CTID: NCT05992311
Phase: Phase 1 Status: Not yet recruiting
Date: 2024-11-06
|
|
|