| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
Na,K-ATPase 抑制作用的研究表明夹竹桃苷的 IC50 (nM) 为 620。夹竹桃苷对 Na,K-ATPase 的抑制表明它可能阻碍钠泵作用从而引起毒性 [1]。未分化的 CaCO-2 细胞对 0.2 至 25 nM 剂量的夹竹桃苷表现出敏感性,IC50 为 8.25 nM。相比之下,当用浓度高达 25 nM 的夹竹桃苷处理时,分化的 CaCO-2 细胞只能实现最大 20% 的生长抑制 [2]。
|
|---|---|
| 体内研究 (In Vivo) |
研究了夹竹桃苷对体内神经胶质瘤生长的影响。因此,SCID或C57BL/6小鼠的右侧纹状体分别移植有人U87MG(5×104)、U251、GBM19(5×105)或鼠(同源)GL261(7.5×104)细胞。 ,十天后,每天腹腔注射夹竹桃苷,连续七天。小鼠和人类的体内神经胶质瘤细胞模型表现出夹竹桃苷导致的肿瘤生长的剂量依赖性减少。高剂量的夹竹桃苷(3 毫克/千克)在两种模型中都是致命的,正如根据已知的啮齿动物致死剂量所预期的那样。通过低于致死剂量(0.3 mg/kg)的夹竹桃苷剂量,注射 U87MG 细胞的小鼠的存活时间从 32.6±1.4 天显着延长至 53.8±9.6 天(n=5-11;p<0.01,对数秩检验) )以及注射 GL261 细胞的小鼠从 23.37±1.2 天到 34.38±3.3 天(n=5-11;p<0.01,对数秩检验)[3]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Pharmacokinetic studies of (3)H oleandrin, a cardiac glycoside component of Anvirzel, were conducted in mice after either an i.v. dose (40 ug/kg) or a p.o. dose (80 ug/kg). Oleandrin was rapidly absorbed after oral dosing (Cmax at 20 min) although the elimination half-life was longer (2.3 +/- 0.5 hr) than that after i.v. dosing (0.4 +/- 0.1 hr). The AUC0-infinity values obtained after i.v. and p.o. dosing were 24.6 +/- 11.1 and 14.4 +/- 4.3 (ng.hr/mL), respectively, resulting in an oral bioavailability of approximately 30%. After i.v. administration, oleandrin concentration in liver was approximately twice that measured in heart or kidney tissue. Oleandrigenin, the aglycone of oleandrin, was also found in these tissues. At 5 min, > 60% of the total radioactivity in liver was due to oleandrin while 28% of the given dose was present as oleandrigenin. Twenty-four hours following injection, 8% of total radioactivity was excreted in urine and contained both oleandrigenin (4.4% of the injected dose) and oleandrin (1.9%). Sixty-six percent of injected radioactivity was found in feces and consisted of oleandrin and oleandrigenin in equal amounts. Uptake of oleandrin in brain after i.p. injection of oleandrin (3 mg/kg) or oleander extract (700 mg/kg) was examined. Measured by LC/MS/MS, oleandrin content in brain was higher following injection of extract than it was with an equivalent dose of oleandrin. The data suggest that components within oleander extract may enhance transport of oleandrin across the blood brain barrier. The toxicity due to an infusion or decoction of N oleander into rabbits was attributed to the oleandrin content in various organs. The heart, stomach, kidneys, and blood contained the greatest oleandrin concentrations, whereas the lung and brain contained none. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Oleandrin is a solid. It is a cardiac glycoside found in plants Nerium oleander (common oleander) and Thevetia peruviana (yellow oleander). HUMAN EXPOSURE AND TOXICITY: Ingestion of either oleandrin results in nausea, vomiting, abdominal pain, diarrhea, dysrhythmias, and hyperkalemia. In poisoning by oleander, mydriasis characteristically accompanies vertigo, convulsions, coma, and bradycardia. Accidental ingestion can cause cardiac arrhythmias and even death. Several cases of fatal and non-fatal poisoning have been reported. ANIMAL STUDIES: Oleander intoxication should be a differential diagnosis for equids with colic in geographic areas where oleander is found, especially when azotemia or cardiac arrhythmias are detected concurrently. ECOTOXICITY STUDIES: For freshwater fish C. punctatus exposure to sub-lethal doses of oleandrin for 24 hr and 96 hr caused significant alteration in the level of total protein, total free amino acid, nucleic acid, glycogen, pyruvate, lactate and enzyme protease, phosphatases, alanine aminotransferase, aspartate aminotransferase and acetylcholinesterase activity in liver and muscle tissues. Interactions Oleandrin plant poisoning is common in children and the plant extract is used in Chinese medicines. The toxicity is due to oleandrin and the deglycosylated metabolite oleandrigenin. Bufalin and cinobufotalin (toad cardiac toxins) are also widely used in Chinese medicines like Chan SU, and Lu-Shen -WU. Severe toxicity from bufalin after consumption of toad soup has been reported. Taking advantage of structural similarities of these toxins with digitoxin, we demonstrated that these compounds can be rapidly detected in blood by the fluorescence polarization immunoassay for digitoxin. The cross reactivities of these compounds with digoxin assay were much lower. For example, when a drug free serum was supplemented with 10 ug/mL of oleandrin, we observed 127.7 ng/mL of digitoxin equivalent but only 2.4 ng/mL of digoxin equivalent concentration. Digibind neutralized all cardiac toxins studied as evidenced by significant fall of free concentrations. When aliquots of serum pool containing 50.0 ug/mL of oleandrin were supplemented with 0, 10.0, 25.0, 50.0, 100, and 200 ug/mL of digibind, the mean free concentrations were 30.6, 23.3, 16.0, 10.7, 7.8 and 5.5 ug/mL respectively. Similarly, with 50.0 ug/mL of oleandrigenin (total concentration: 36.2 ng/mL), the free concentration was 14.5 ng/mL digitoxin equivalent in the absence of digibind and 5.4 ng/mL in the presence of 200 microg/mL of digibind. In another specimen containing 500 ng/mL bufalin (total concentration: 156.9 ng/mL), the free concentration was 8.6 ng/mL in the absence of digibind and none detected in the presence of 100.0 ug/mL digibind. Because such neutralization may also occur in vivo, digibind may be useful in treating patients exposed to these toxins. Considering the potential role of interleukin-8 (IL-8) in inflammation, angiogenesis, tumorogenesis, and metastasis, and the involvement of different cell types especially neutrophils and macrophages in those processes, the regulation of IL-8-mediated biological responses is important. In this report we provide evidences that oleandrin, a cardiac glycoside potentially inhibited IL-8-, formyl peptide (FMLP)-, EGF-, or nerve growth factor (NGF)-, but not IL-1- or TNF-induced NF-kappaB activation in macrophages. Oleandrin inhibited IL-8-, but not TNF-induced NF-kappaB-dependent genes expression. Oleandrin inhibited the binding of IL-8, EGF, or NGF, but not IL-1 or TNF. It decreased almost 79% IL-8 binding without altering affinity towards IL-8 receptors and this inhibition of IL-8 binding was observed in isolated membrane. The IL-8, anti-IL-8Rs antibodies, or protease inhibitors were unable to protect oleandrin-mediated inhibition of IL-8 binding. Phospholipids significantly protected oleandrin-mediated inhibition of IL-8 binding thereby restoring IL-8-induced NF-kappaB activation. Oleandrin altered the membrane fluidity as detected by microviscosity parameter and a decrease in diphenylhexatriene, a lipid binding fluorophore binding in a dose-dependent manner. Overall, our results suggest that oleandrin inhibits IL-8-mediated biological responses in diverse cell types by modulating IL-8Rs through altering membrane fluidity and microviscosity. The study might help to regulate IL-8-mediated biological responses involved in inflammation, metastasis, and neovascularization. Oleander (Nerium oleander) poisoning is a common problem found in many parts of the world. The oleander toxicity is due to oleandrin and its aglycone metabolite oleandrigenin. Activated charcoal is a useful gastrointestinal decontamination agent that limits the absorption of ingested toxins. A relatively new clay product, Bio-Sponge, containing di-tri-octahedral smectite as the active ingredient, is also recommended for adsorbing bacterial toxins in the gastrointestinal tract. Bio-Sponge has been used to prevent gastrointestinal absorption of oleander toxins in livestock but the efficacy of activated charcoal and Bio-Sponge for adsorbing oleandrin and oleandrigenin has not yet been studied. An in vitro experiment to compare the efficacy of three commercially available adsorbents was performed. The adsorbents include Bio-Sponge, ToxiBan granules, and a generic grade activated charcoal. ToxiBan granules have the highest adsorptive capacity, followed by the generic grade activated charcoal, and finally, Bio-Sponge. Bio-Sponge did not adsorb oleandrin and oleandrigenin at concentrations that are expected to be present in the gastrointestinal tract of poisoned animals. On the basis of this in vitro study, products containing activated charcoal are more effective for binding oleander toxins and providing gastrointestinal decontamination than products containing di-tri-octahedral smectite. However, the ability of these adsorbents to alter the clinical outcome in oleander-poisoned animals or humans is yet to be evaluated. Cardiac glycosides such as digitoxin and ouabain have previously been shown to be selectively cytotoxic to tumor as opposed to normal cells. Moreover, this class of agents has also been shown to act as potent radiosensitizers. In the present study we explored the relative radiosensitization potential of oleandrin, a cardiac glycoside contained within the plant extract known as Anvirzel that recently underwent a Phase I trial as a novel drug for anticancer therapy. The data show that oleandrin produces an enhancement of sensitivity of PC-3 human prostate cells to radiation; at a cell survival of 0.1, the enhancement factor was 1.32. The magnitude of radiosensitization depended on duration of exposure of cells to drug prior to radiation treatment. While a radiosensitizing effect of oleandrin was evident with only 1 hr of cell exposure to drug, the effect greatly increased with 24 hr oleandrin pretreatment. Susceptibility of PC-3 cells to oleandrin and radiation-induced apoptosis was dependent on activation of caspase-3. Activation was greatest when cells were exposed simultaneously to oleandrin and radiation. Inhibition of caspase-3 activation with Z-DEVD-FMK abrogated the oleandrin-induced enhancement of radiation response suggesting that both oleandrin and radiation share a caspase-3 dependent mechanism of apoptosis in the PC-3 cell line. Ceramide (N-acetyl-D-sphingosine), a second messenger for cell signaling, induces transcription factors, like nuclear factor-kappa B (NF-kappa B), and activator protein-1 (AP-1) and is involved in inflammation and apoptosis. Agents that can suppress these transcription factors may be able to block tumorigenesis and inflammation. Oleandrin (trans-3,4',5-trihydroxystilbene), a polyphenolic cardiac glycoside derived from the leaves of Nerium oleander, has been used in the treatment of cardiac abnormalities in Russia and China for years. We investigated the effect of oleandrin on NF-kappa B and AP-1 activation and apoptosis induced by ceramide. Oleandrin blocked ceramide-induced NF-kappa B activation. Oleandrin-mediated suppression of NF-kappa B was not restricted to human epithelial cells; it was also observed in human lymphoid, insect, and murine macrophage cells. The suppression of NF-kappa B coincided with suppression of AP-1. Ceramide-induced reactive intermediates generation, lipid peroxidation, cytotoxicity, caspase activation, and DNA fragmentation were potentiated by oleandrin. Oleandrin did not show its activity in primary cells. Oleandrin's anticarcinogenic, anti-inflammatory, and growth-modulatory effects may thus be partially ascribed to the inhibition of activation of NF-kappa B and AP-1 and potentiation of apoptosis. Non-Human Toxicity Values LD50 Cat iv 300 ug/kg |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
The purpose of this study was to examine the mechanism(s) and differential cell-killing effects of Anvirzel, an extract of oleander (Nerium oleander; family-Apocynaceae), and its derivative compound Oleandrin on human, canine and murine tumor cells. Cells received different concentrations of Anvirzel (1.0 ng/mL to 500 ug/mL) or Oleandrin (0.01 ng/ml to 50 ug/mL) in both continuously treated and pulse-treated/recovery cultures. The cytotoxicity of these compounds was then determined. Both Anvirzel and Oleandrin were able to induce cell killing in human cancer cells, but not in murine cancer cells; the cell-killing potency of Oleandrin was greater than that of Anvirzel. Canine oral cancer cells treated with Anvirzel showed intermediate levels of response, with some abnormal metaphases and cell death resulting from the treatment. From these results we conclude that Anvirzel and Oleandrin act in a species-specific manner, and while testing the effectiveness of a new compound for cancer treatment, one must use not only murine but a variety of cancer cells, including those of human origin. /EXPL THER/ Oleandrin, derived from the leaves of Nerium oleander, has been shown to possess anti-inflammatory and tumor cell growth-inhibitory effects. Here, we provide evidence that oleandrin could possess anti-tumor promoting effects. We determined the effect of topical application of oleandrin to CD-1 mice against l2-O-tetradecanoylphorbol-13-acetate (TPA), a widely studied skin tumor promoter, -induced conventional and novel markers of skin tumor promotion. Topical application of oleandrin (2 mg per mouse) 30 min before TPA (3.2 nmol per mouse) application onto the skin afforded significant inhibition, in a time-dependent manner, against TPA-mediated increase in cutaneous edema and hyperplasia, epidermal ornithine decarboxylase (ODC) activity and ODC and cyclooxgenase-2 (COX-2) protein expression. In search for novel markers of skin tumor promotion, we found that TPA application to mouse skin resulted, as an early event, in an increased expression of phosphatidyinositol 3-kinase (PI3K), phosphorylation of Akt at threonine308 and activation of nuclear factor kappa B (NF-kappaB). Topical application of oleandrin before TPA application to mouse skin resulted in significant reduction in TPA-induced expression of PI3K and phosphorylation of Akt, and inhibition of NF-kappaB activation. NF-kappaB is a eukaryotic transcription factor that is critically involved in regulating the expression of specific genes that participate in inflammation, apoptosis and cell proliferation. Employing Western blot analysis, we found that oleandrin application to mouse skin resulted in inhibition of TPA-induced activation of NF-kappaB, IKKalpha and phosphorylation and degradation of IkappaBalpha. Our data suggest that oleandrin could be a useful anti-tumor promoting agent because it inhibits several biomarkers of TPA-induced tumor promotion in an in vivo animal model. One might envision the use of chemopreventive agents such as oleandrin in an emollient or patch for chemoprevention or treatment of skin cancer. /EXPL THER/ NF-kappaB is a ubiquitous and well-characterized protein responsible for the regulation of complex phenomena, with a pivotal role in controlling cell signaling in the body under certain physiological and pathological conditions. Among other functions, NF-kappaB controls the expression of genes encoding the pro-inflammatory cytokines (e. g., IL-1, IL-2, IL-6, TNF-alpha, etc.), chemokines (e. g., IL-8, MIP-1alpha, MCP1, RANTES, eotaxin, etc.), adhesion molecules (e. g., ICAM, VCAM, E-selectin), inducible enzymes (COX-2 and iNOS), growth factors, some of the acute phase proteins, and immune receptors, all of which play critical roles in controlling most inflammatory processes. Since NF-kappaB represents an important and very attractive therapeutic target for drugs to treat many inflammatory diseases, including arthritis, asthma, and the auto-immune diseases, most attention has been paid in the last decade to the identification of compounds that selectively interfere with this pathway. Recently, a great number of plant-derived substances have been evaluated as possible inhibitors of the NF-kappaB pathway. These include a wide range of compound classes, such as lignans (manassantins, (+)-saucernetin, (-)-saucerneol methyl ether), sesquiterpenes (costunolide, parthenolide, celastrol, celaphanol A), diterpenes (excisanin, kamebakaurin), triterpenes (avicin, oleandrin), polyphenols (resveratrol, epigallocatechin gallate, quercetin), etc. In this mini-review we will discuss the medicinal chemistry of these compounds with regards to the NF-kappaB inhibition. /EXPL THER/ The treatment of cancer with chemotherapeutic agents and radiation has two major problems: time-dependent development of tumor resistance to therapy (chemoresistance and radioresistance) and nonspecific toxicity toward normal cells. Many plant-derived polyphenols have been studied intently for their potential chemopreventive properties and are pharmacologically safe. These compounds include genistein, curcumin, resveratrol, silymarin, caffeic acid phenethyl ester, flavopiridol, emodin, green tea polyphenols, piperine, oleandrin, ursolic acid, and betulinic acid. Recent research has suggested that these plant polyphenols might be used to sensitize tumor cells to chemotherapeutic agents and radiation therapy by inhibiting pathways that lead to treatment resistance. These agents have also been found to be protective from therapy-associated toxicities. How these polyphenols protect normal cells and sensitize tumor cells to treatment is discussed in this review. /EXPL THER/ The principal active constituent of the botanical drug candidate PBI-05204, a supercritical CO(2) extract of Nerium oleander, is the cardiac glycoside oleandrin. PBI-05204 shows potent anticancer activity and is currently in phase I clinical trial as a treatment for patients with solid tumors. We have previously shown that neriifolin, which is structurally related to oleandrin, provides robust neuroprotection in brain slice and whole animal models of ischemic injury. However, neriifolin itself is not a suitable drug development candidate and the FDA-approved cardiac glycoside digoxin does not cross the blood-brain barrier. We report here that both oleandrin as well as the full PBI-05204 extract can also provide significant neuroprotection to neural tissues damaged by oxygen and glucose deprivation as occurs in ischemic stroke. Critically, we show that the neuroprotective activity of PBI-05204 is maintained for several hours of delay of administration after oxygen and glucose deprivation treatment. We provide evidence that the neuroprotective activity of PBI-05204 is mediated through oleandrin and/or other cardiac glycoside constituents, but that additional, non-cardiac glycoside components of PBI-05204 may also contribute to the observed neuroprotective activity. Finally, we show directly that both oleandrin and the protective activity of PBI-05204 are blood brain barrier penetrant in a novel model for in vivo neuroprotection. Together, these findings suggest clinical potential for PBI-05204 in the treatment of ischemic stroke and prevention of associated neuronal death. |
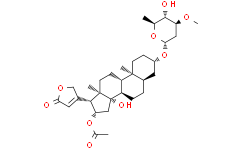
| 分子式 |
C32H48O9
|
|---|---|
| 分子量 |
576.72
|
| 精确质量 |
576.329
|
| CAS号 |
465-16-7
|
| PubChem CID |
11541511
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.26
|
| 沸点 |
693.7±55.0 °C at 760 mmHg
|
| 熔点 |
250ºC
|
| 闪点 |
217.2±25.0 °C
|
| 蒸汽压 |
0.0±4.9 mmHg at 25°C
|
| 折射率 |
1.567
|
| LogP |
2.3
|
| tPSA |
120.75
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
41
|
| 分子复杂度/Complexity |
1080
|
| 定义原子立体中心数目 |
13
|
| SMILES |
C[C@H]1[C@@H]([C@H](C[C@@H](O1)O[C@H]2CC[C@]3([C@@H](C2)CC[C@@H]4[C@@H]3CC[C@]5([C@@]4(C[C@@H]([C@@H]5C6=CC(=O)OC6)OC(=O)C)O)C)C)OC)O
|
| InChi Key |
JLPDBLFIVFSOCC-XYXFTTADSA-N
|
| InChi Code |
InChI=1S/C32H48O9/c1-17-29(35)24(37-5)14-27(39-17)41-21-8-10-30(3)20(13-21)6-7-23-22(30)9-11-31(4)28(19-12-26(34)38-16-19)25(40-18(2)33)15-32(23,31)36/h12,17,20-25,27-29,35-36H,6-11,13-16H2,1-5H3/t17-,20+,21-,22-,23+,24-,25-,27-,28-,29-,30-,31+,32-/m0/s1
|
| 化学名 |
[(3S,5R,8R,9S,10S,13R,14S,16S,17R)-14-hydroxy-3-[(2R,4S,5S,6S)-5-hydroxy-4-methoxy-6-methyloxan-2-yl]oxy-10,13-dimethyl-17-(5-oxo-2H-furan-3-yl)-1,2,3,4,5,6,7,8,9,11,12,15,16,17-tetradecahydrocyclopenta[a]phenanthren-16-yl] acetate
|
| 别名 |
PBI-05204 PBI 05204 PBI05204
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~173.39 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.33 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.33 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.33 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7339 mL | 8.6697 mL | 17.3394 mL | |
| 5 mM | 0.3468 mL | 1.7339 mL | 3.4679 mL | |
| 10 mM | 0.1734 mL | 0.8670 mL | 1.7339 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。