| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
Autotaxin (IC50 = 2.8 nM)
PF-8380 is a selective inhibitor of autotaxin (ATX, also named ectonucleotide pyrophosphatase/phosphodiesterase 2, ENPP2); the IC50 for recombinant human ATX is 25 nM, and the Ki value for human ATX is 10 nM [1] PF-8380 has no significant inhibitory activity (IC50 > 10 μM) against other phosphodiesterases (PDE1-PDE11) or lysophospholipases [1] |
|---|---|
| 体外研究 (In Vitro) |
此外,PF-8380 还能抑制大鼠自分泌运动因子(FS-3 的底物),IC50 为 1.16 nM。当胎儿成纤维细胞产生的酶与作为底物的溶血磷脂酰胆碱 (LPC) 结合时,PF-8380 的功效保持不变。当用 PF-8380 以 101 nM 的 IC50 处理人全血两小时时,自分泌运动因子受到抑制 [1]。溶血磷脂酶 D (lysoPLD) 活性由自分泌运动因子 (ATX) 发挥作用,该酶催化溶血磷脂酰胆碱 (LPC) 转化为溶血磷脂酸 (LPA)。对 GL261 和 U87-MG 细胞应用 1 μM PF-8380 作为预处理后,将它们暴露于 4 Gy 的辐射下,导致克隆存活率下降,迁移减少(GL261 中为 33%;P=0.002 和 17.9%) U87-MG 中;P=0.012),侵袭减少(GL261 中 35.6%;P=0.0037;U87-MG 中 31.8%;P=0.002),并减弱辐射诱导的 Akt 磷酸化 [2]。
1. ATX活性抑制:PF-8380(0.1–100 nM)可浓度依赖性抑制体外重组人源和鼠源ATX的活性,100 nM浓度下可完全抑制人源ATX;同时能减少人血浆中溶血磷脂酰胆碱(LPC)向溶血磷脂酸(LPA)的转化,IC50为30 nM[1] 2. 胶质母细胞瘤细胞系:PF-8380(1–50 μM)可剂量依赖性抑制人源(U87、U251)和鼠源(GL261)胶质母细胞瘤细胞增殖,72小时处理后的IC50分别为12 μM(U87)、15 μM(U251)和10 μM(GL261);还能增强这些细胞的放射敏感性,5 μM浓度下对U87细胞的放射增强比(RER)为1.8[2] 3. 胶质母细胞瘤克隆形成与凋亡:PF-8380(5 μM)使U87细胞的克隆形成率降低60%,并增加辐射诱导的凋亡(4 Gy照射后Annexin V+/PI+细胞比例从12%升至38%);western blot检测显示剪切型caspase-3上调,抗凋亡蛋白Bcl-2下调[2] 4. 肺移植纤维化相关细胞:PF-8380(10 μM)可抑制LPA诱导的人肺成纤维细胞(HLFs)中β-连环蛋白(β-catenin)的Ser552位点磷酸化及核转位,使纤维化标志物(α-SMA、I型胶原)的表达分别降低55%和48%(qPCR检测),并抑制HLFs增殖(IC50=8 μM)[3] 5. LPA信号通路抑制:PF-8380(5–20 μM)可阻断胶质母细胞瘤和肺成纤维细胞中LPA诱导的ERK1/2和AKT磷酸化,表明其能抑制LPA-Gi/PI3K/ERK信号轴[2][3] |
| 体内研究 (In Vivo) |
PF-8380 的药代动力学特性在 24 小时内以 1 mg/kg 静脉注射剂量和 1 至 100 mg/kg 口服剂量进行评估。 PF-8380的有效t1/2为1.2小时,稳态分布体积为3.2L/kg,平均清除率为31mL/min/kg。口服生物利用度中等,范围为 43% 至 83%。随着单次口服剂量的增加,血浆浓度也会升高;然而,Cmax的增加速率小于与10至100mg/kg的剂量成比例,但与1至10mg/kg的剂量大致成比例。高达 100 mg/kg 时,PF-8380 的暴露大致与剂量成比例且呈线性,如曲线下面积所示。采集后立即检测血浆 C16:0、C18:0 和 C20:0 LPA 的量。使用 3 mg/kg 剂量时,LPA 水平最大下降出现在 0.5 小时,24 小时内,所有 LPA 均恢复至或超过基线 [1]。使用 10 mg/kg PF-8380 治疗后,肿瘤相关血管分布适度增加 20%(P=0.497)。在 4 Gy 照射前 45 分钟,PF-8380 治疗使接受治疗的小鼠的血管分布与对照组相比减少了约 48% (P=0.031),而仅接受放射治疗的小鼠则减少了 65% (P=0.011)[2]。
1. 大鼠炎症模型:在角叉菜胶诱导的大鼠足肿胀模型中,口服PF-8380(30 mg/kg)后4小时,血浆LPA水平降低70%,足跖炎症部位LPA水平降低65%;与溶媒组相比,足肿胀程度减轻50%,髓过氧化物酶(MPO,中性粒细胞浸润标志物)活性降低60%[1] 2. 胶质母细胞瘤异种移植模型:在U87胶质母细胞瘤裸鼠异种移植模型中,腹腔注射PF-8380(20 mg/kg,每日一次)联合分次放疗(2 Gy/天,连续5天),肿瘤生长抑制率(TGI)达80%,而单纯放疗的TGI为35%,单纯PF-8380治疗的TGI为25%;联合治疗还将小鼠中位生存期从单纯放疗的32天延长至58天[2] 3. 小鼠肺移植纤维化模型:在小鼠原位肺移植模型中,每日两次口服PF-8380(15 mg/kg),连续28天,肺移植体纤维化评分从3.5降至1.2(评分范围0–4),胶原沉积减少62%(Masson三色染色),肺组织中β-连环蛋白的核蓄积减少70%(免疫荧光检测)[3] 4. 体内LPA水平调控:PF-8380(30 mg/kg,口服)使肺移植小鼠支气管肺泡灌洗液(BALF)中的LPA水平降低55%,并下调肺组织中LPA受体(LPA1、LPA2)的表达[3] |
| 酶活实验 |
ATX ELISA和ATX活性测定。[3]
BOS和非BOS细胞系在60mm培养皿中培养直至融合。细胞用PBS洗涤一次,然后血清饥饿24小时。收集无血清上清液,并根据制造商的方案用人ENPP-2/Autotaxin Quantikine ELISA试剂盒测量ATX水平。使用SpectraMax M3多模式微孔板读数器测量450nm处的吸光度。对于ATX活性,收集细胞上清液,在4°C下以17000 g离心10分钟,以沉淀漂浮的细胞或碎片,并用带Ultracel-3膜的Amicon Ultra-4离心过滤器浓缩至原始体积的八分之一。在测量蛋白质浓度后,用荧光磷脂ATX底物FS-3对等量的总蛋白质进行ATX活性测定。简而言之,将30μl上清液和40μl FS-3溶液(含有5μM FS-3、140 mM NaCl、5 mM KCl、1 mM CaCl2、1 mM MgCl2、50 mM Tris-HCl pH 8.0和1 mg/ml BSA)混合并装载到Costar 96孔黑色壁透明底板上。分别在485nm和528nm的激发和发射波长下,使用SpectraMax M3多模微孔板读数器测量样品的荧光。 对于肺裂解物中的ATX活性测定,将20μl同种异体裂解物和40μl FS-3溶液混合,并对安慰剂和PF-8380治疗的肺同种异体移植物进行类似的ATX活动测定。 1. ATX活性荧光实验流程:将重组人ATX与系列浓度的PF-8380及荧光底物FS-3(人工合成的LPC类似物)共同孵育于96孔板中,37°C孵育1小时后,检测荧光强度(激发光485 nm,发射光535 nm)以反映LPA生成量;绘制剂量-反应曲线,计算ATX抑制的IC50和Ki值[1] 2. 血浆LPA生成实验流程:将人血浆与PF-8380(0–100 nM)预孵育30分钟,加入100 μM LPC作为底物,37°C孵育2小时后,通过液相色谱-串联质谱(LC-MS/MS)定量血浆中LPA浓度,评估PF-8380对内源性ATX的抑制效果[1] |
| 细胞实验 |
共培养克隆存活试验[2]
将HUVEC(1.0×106)和bEnd.3细胞(1.0×10^6)铺在100mm板上,24小时后,将U87-MG(2×10^7)和GL261(2×106)细胞铺在transwell插入物上。共培养24小时后,在用0、2、4、6或8 Gy照射之前,用1μM的PF-8380或载体对照DMSO处理细胞45分钟。与PF-8380或DMSO共培养处理后,将计算出的U87-MG和GL261细胞数量进行铺板,以使铺板效率正常化。孵育7至10天后,用70%乙醇固定平板,并用1%亚甲基蓝染色。通过在显微镜下观察平板,对由>50个细胞组成的菌落进行计数。存活分数计算为(集落数/铺板细胞数)/(相应对照的集落数除以铺板细胞数来)。通过对计算D0和n的α/β模型进行曲线拟合来分析生存曲线。 细胞迁移的伤口愈合/划痕分析[2] 将GL261或U87-MG细胞一式三份铺在6cm的板上,并使其生长至70%融合。用无菌200μL移液管尖端刮擦半融合细胞层,以形成没有细胞的划痕,并用PBS洗涤平板一次,以去除非粘附细胞和碎片。对于放射增敏药物研究,在用4 Gy照射之前,用1μMPF-8380或DMSO处理细胞45分钟,然后在37°C的5%CO2中孵育。监测对照板的细胞迁移(20-24小时)。细胞用70%乙醇固定,用1%亚甲基蓝染色。为了量化迁移,对划痕区域中三个随机选择的高功率场(HPF)中的细胞进行计数,并对周围细胞密度进行归一化。计算每个治疗组的平均值和标准误差。 肿瘤经口侵袭试验[2] 肿瘤经口基质凝胶侵袭试验以前曾用于帮助定量肿瘤与内皮的相互作用和转移。GL261(1.0×106个细胞/孔)或U87-MG(0.6×106个电池/孔)悬浮在无血清培养基中,并加入到带有8μm孔的基质涂层聚碳酸酯膜过滤器的上室(插入物)中。将500微升新鲜培养基加入底部腔室。对于放射增敏药物研究,在用4 Gy照射之前,两个腔室都用载体DMSO或1μMPF-8380处理45分钟。36小时后,用湿棉签去除膜插入物上腔室中的剩余细胞。将粘附在穿过基质凝胶侵入的transwell插入膜外表面上的细胞用100%甲醇固定并染色。使用Image J软件对每个样本的7-10个HPF中的侵袭细胞进行计数,并计算每个HPF中侵袭细胞的平均数量。计算每个治疗组的平均值和标准误差。 1. 胶质母细胞瘤细胞增殖实验:将U87、U251和GL261细胞以5×10³个/孔接种于96孔板,加入PF-8380(0.1–100 μM)处理72小时;通过CCK-8法检测450 nm处吸光度评估细胞活力,计算增殖抑制的IC50[2] 2. 放射敏感性克隆形成实验:将胶质母细胞瘤细胞以500个/孔接种于6孔板,用PF-8380(0–10 μM)处理24小时后,接受0–8 Gy电离辐射;培养14天后,用结晶紫染色并计数克隆数,计算存活分数以确定放射增强比[2] 3. 胶质母细胞瘤凋亡检测实验:用PF-8380(5 μM)和/或4 Gy辐射处理U87细胞48小时;经Annexin V-FITC和PI染色后,通过流式细胞术定量凋亡细胞;提取总蛋白,通过western blot检测剪切型caspase-3、Bcl-2和Bax的表达[2] 4. 肺成纤维细胞功能实验:用PF-8380(0–20 μM)和1 μM LPA处理人肺成纤维细胞(HLFs)48小时;通过BrdU掺入实验评估细胞增殖,qPCR检测α-SMA和I型胶原的mRNA水平(以GAPDH为内参);分离细胞核和细胞质组分,通过western blot分析β-连环蛋白的亚细胞定位[3] 5. LPA信号western blot实验:用PF-8380(5–20 μM)处理胶质母细胞瘤和肺成纤维细胞1小时,再加入1 μM LPA刺激15分钟;提取总蛋白,通过SDS-PAGE和免疫印迹检测p-ERK1/2、总ERK1/2、p-AKT、总AKT和β-肌动蛋白的表达[2][3] |
| 动物实验 |
Mice, treatment, and tumor growth delay [2]
All animal procedures used in this study were approved by IACUC. Handling of animals and housing was followed as per DCM guidelines. GL261 cells (1 × 106) were injected into the right hind limb of nude mice. Once tumors were palpable the mice were serpentine sorted into groups of six to seven animals representing similar distributions of tumor sizes (range = 240 mm3). Tumor bearing mice were injected intraperitoneally with vehicle (DMSO) or PF-8380 at 10 mg/kg body weight once daily for five consecutive days. Forty five minutes after drug injection, mice were anesthetized with isoflurane and positioned in the RS2000 irradiator. They were then irradiated with 2 Gy daily for five consecutive days for a total of 10 Gy. Lead blocks (10 mm thick) were used to shield the head, thorax, and abdomen. Tumor size was monitored longitudinally using an external traceable digital caliper. Oral gavage was performed in a containment room of the animal facility. PF-8380 and AM095 were dissolved in PEG 400 at a concentration of 6 mg/ml. Body weights of animals were measured daily. Treatment with PF-8380 or AM095 was administered by oral gavage twice daily at a dosage of 30 mg/kg body weight starting from day 14 after lung transplantation. Placebo-treated mice were given vehicle (PEG 400) via oral gavage ingestion. On day 40 after lung transplantation, mice were sacrificed, and lung allografts were harvested for Western blotting, hydroxyproline assay, or immunohistochemistry. 1. Rat carrageenan-induced paw edema model: Male Sprague-Dawley rats were randomly divided into vehicle and PF-8380 groups (n=6 per group); PF-8380 was dissolved in 10% DMSO, 40% PEG400, and 50% normal saline, and administered orally at 10, 30, and 50 mg/kg; 1 hour later, 100 μL of 1% carrageenan was injected into the right hind paw to induce inflammation; paw volume was measured at 1, 4, and 8 hours post-carrageenan injection, and plasma/paw tissues were collected for LPA and MPO activity analysis [1] 2. U87 glioblastoma xenograft model: Female BALB/c nude mice (6–8 weeks old) were subcutaneously inoculated with U87 cells (1×10⁷) into the flank; when tumors reached 100 mm³, mice were randomized into four groups: vehicle, PF-8380 monotherapy (20 mg/kg, intraperitoneal injection, once daily), radiation monotherapy (2 Gy/day for 5 days), and combination therapy; PF-8380 was dissolved in 5% DMSO, 20% Cremophor EL, and 75% normal saline; tumor volume was measured twice weekly, and survival was monitored for 80 days [2] 3. Mouse lung allograft fibrosis model: C57BL/6 mice (recipients) received orthotopic left lung transplants from BALB/c mice (donors); PF-8380 was formulated in 0.5% CMC-Na solution and administered by oral gavage at 15 mg/kg twice daily for 28 days starting from the day of transplantation; vehicle-treated mice received 0.5% CMC-Na alone; at study end, lung tissues were collected for histopathological scoring, Masson’s trichrome staining, and immunofluorescence detection of β-catenin [3] |
| 药代性质 (ADME/PK) |
1. Oral bioavailability: PF-8380 has an oral bioavailability of 45% in rats after oral administration of 30 mg/kg [1]
2. Plasma pharmacokinetics: In rats, oral PF-8380 (30 mg/kg) reached a maximum plasma concentration (Cmax) of 1.2 μM at 1.5 hours post-administration, with a plasma half-life (t1/2) of 4.2 hours; the area under the curve (AUC0-24h) was 8.5 μM·h [1] 3. Tissue distribution: PF-8380 accumulates in inflamed tissues (rat paw: 2.8 μM) and lung tissues (mouse lung: 3.1 μM) at 4 hours after oral administration, with a tissue/plasma ratio of 2.3 (paw) and 2.6 (lung) [1][3] 4. Metabolism and excretion: PF-8380 is primarily metabolized in the liver by glucuronidation; approximately 60% of the drug is excreted via feces and 30% via urine within 24 hours, with unchanged drug accounting for 15% of total excretion [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
1. Acute toxicity: PF-8380 was well tolerated in mice and rats at oral doses up to 200 mg/kg and intraperitoneal doses up to 100 mg/kg, with no mortality or severe clinical signs (weight loss, lethargy) observed [1][2]
2. Subchronic toxicity: In a 28-day rat study, oral PF-8380 (10, 30, 100 mg/kg/day) caused mild diarrhea only at 100 mg/kg, with no significant changes in hematological (RBC, WBC, platelets) or serum biochemical (ALT, AST, creatinine) parameters [1] 3. Plasma protein binding: PF-8380 has a plasma protein binding rate of 92% in human plasma, 90% in rat plasma, and 88% in mouse plasma (measured by ultrafiltration) [1] 4. Organ toxicity: Histological analysis of liver, kidney, and lung tissues from PF-8380-treated animals showed no signs of inflammation, necrosis, or fibrosis [1][3] 5. Drug-drug interactions: In vitro studies showed that PF-8380 does not inhibit CYP450 isoforms (CYP3A4, CYP2C9, CYP2D6) at therapeutic concentrations (up to 1 μM) [1] |
| 参考文献 |
|
| 其他信息 |
Autotaxin is the enzyme responsible for the production of lysophosphatidic acid (LPA) from lysophosphatidyl choline (LPC), and it is up-regulated in many inflammatory conditions, including but not limited to cancer, arthritis, and multiple sclerosis. LPA signaling causes angiogenesis, mitosis, cell proliferation, and cytokine secretion. Inhibition of autotaxin may have anti-inflammatory properties in a variety of diseases; however, this hypothesis has not been tested pharmacologically because of the lack of potent inhibitors. Here, we report the development of a potent autotaxin inhibitor, PF-8380 [6-(3-(piperazin-1-yl)propanoyl)benzo[d]oxazol-2(3H)-one] with an IC(50) of 2.8 nM in isolated enzyme assay and 101 nM in human whole blood. PF-8380 has adequate oral bioavailability and exposures required for in vivo testing of autotaxin inhibition. Autotaxin's role in producing LPA in plasma and at the site of inflammation was tested in a rat air pouch model. The specific inhibitor PF-8380, dosed orally at 30 mg/kg, provided >95% reduction in both plasma and air pouch LPA within 3 h, indicating autotaxin is a major source of LPA during inflammation. At 30 mg/kg PF-8380 reduced inflammatory hyperalgesia with the same efficacy as 30 mg/kg naproxen. Inhibition of plasma autotaxin activity correlated with inhibition of autotaxin at the site of inflammation and in ex vivo whole blood. Furthermore, a close pharmacokinetic/pharmacodynamic relationship was observed, which suggests that LPA is rapidly formed and degraded in vivo. PF-8380 can serve as a tool compound for elucidating LPA's role in inflammation. [1]
\n\nPurpose: Glioblastoma multiforme (GBM) is an aggressive primary brain tumor that is radio-resistant and recurs despite aggressive surgery, chemo, and radiotherapy. Autotaxin (ATX) is over expressed in various cancers including GBM and is implicated in tumor progression, invasion, and angiogenesis. Using the ATX specific inhibitor, PF-8380, we studied ATX as a potential target to enhance radiosensitivity in GBM.\n\nMethods and materials: Mouse GL261 and Human U87-MG cells were used as GBM cell models. Clonogenic survival assays and tumor transwell invasion assays were performed using PF-8380 to evaluate role of ATX in survival and invasion. Radiation dependent activation of Akt was analyzed by immunoblotting. Tumor induced angiogenesis was studied using the dorsal skin fold model in GL261. Heterotopic mouse GL261 tumors were used to evaluate the efficacy of PF-8380 as a radiosensitizer.\n\nResults: Pre-treatment of GL261 and U87-MG cells with 1 μM PF-8380 followed by 4 Gy irradiation resulted in decreased clonogenic survival, decreased migration (33% in GL261; P = 0.002 and 17.9% in U87-MG; P = 0.012), decreased invasion (35.6% in GL261; P = 0.0037 and 31.8% in U87-MG; P = 0.002), and attenuated radiation-induced Akt phosphorylation. In the tumor window model, inhibition of ATX abrogated radiation induced tumor neovascularization (65%; P = 0.011). In a heterotopic mouse GL261 tumors untreated mice took 11.2 days to reach a tumor volume of 7000 mm(3), however combination of PF-8380 (10 mg/kg) with irradiation (five fractions of 2 Gy) took more than 32 days to reach a tumor volume of 7000 mm(3).\n\nConclusion: Inhibition of ATX by PF-8380 led to decreased invasion and enhanced radiosensitization of GBM cells. Radiation-induced activation of Akt was abrogated by inhibition of ATX. Furthermore, inhibition of ATX led to diminished tumor vascularity and delayed tumor growth. These results suggest that inhibition of ATX may ameliorate GBM response to radiotherapy. [2] \n\nTissue fibrosis is the primary cause of long-term graft failure after organ transplantation. In lung allografts, progressive terminal airway fibrosis leads to an irreversible decline in lung function termed bronchiolitis obliterans syndrome (BOS). Here, we have identified an autocrine pathway linking nuclear factor of activated T cells 2 (NFAT1), autotaxin (ATX), lysophosphatidic acid (LPA), and β-catenin that contributes to progression of fibrosis in lung allografts. Mesenchymal cells (MCs) derived from fibrotic lung allografts (BOS MCs) demonstrated constitutive nuclear β-catenin expression that was dependent on autocrine ATX secretion and LPA signaling. We found that NFAT1 upstream of ATX regulated expression of ATX as well as β-catenin. Silencing NFAT1 in BOS MCs suppressed ATX expression, and sustained overexpression of NFAT1 increased ATX expression and activity in non-fibrotic MCs. LPA signaling induced NFAT1 nuclear translocation, suggesting that autocrine LPA synthesis promotes NFAT1 transcriptional activation and ATX secretion in a positive feedback loop. In an in vivo mouse orthotopic lung transplant model of BOS, antagonism of the LPA receptor (LPA1) or ATX inhibition decreased allograft fibrosis and was associated with lower active β-catenin and dephosphorylated NFAT1 expression. Lung allografts from β-catenin reporter mice demonstrated reduced β-catenin transcriptional activation in the presence of LPA1 antagonist, confirming an in vivo role for LPA signaling in β-catenin activation.[3] 1. PF-8380 is a first-generation small-molecule ATX inhibitor developed by Pfizer, designed to block LPA production by inhibiting ATX, a key enzyme in the LPA signaling pathway [1] 2. The mechanism of action of PF-8380 involves competitive binding to the catalytic domain of ATX, preventing the hydrolysis of LPC to LPA, thereby inhibiting LPA-mediated signaling (Gi/PI3K/ERK, β-catenin) involved in inflammation, cancer progression, and fibrosis [1][2] [3] 3. PF-8380 is being investigated for the treatment of inflammatory diseases, glioblastoma (in combination with radiation), and lung allograft fibrosis; it is in preclinical development with no clinical trials or FDA warning information reported [1][2] [3] 4. PF-8380 exhibits tissue-specific LPA reduction, with greater efficacy at the site of inflammation/fibrosis than in systemic circulation, indicating a favorable therapeutic profile [1][3] |
| 分子式 |
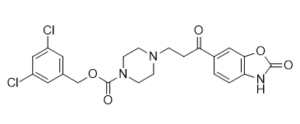
C22H21CL2N3O5
|
|---|---|
| 分子量 |
478.3252
|
| 精确质量 |
477.085
|
| 元素分析 |
C, 55.24; H, 4.43; Cl, 14.82; N, 8.78; O, 16.72
|
| CAS号 |
1144035-53-9
|
| 相关CAS号 |
PF-8380 hydrochloride;2070015-01-7
|
| PubChem CID |
25265312
|
| 外观&性状 |
White to light brown solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 折射率 |
1.616
|
| LogP |
3.63
|
| tPSA |
95.85
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
32
|
| 分子复杂度/Complexity |
693
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1C([H])=C(C([H])=C(C=1[H])C([H])([H])OC(N1C([H])([H])C([H])([H])N(C([H])([H])C([H])([H])C(C2C([H])=C([H])C3=C(C=2[H])OC(N3[H])=O)=O)C([H])([H])C1([H])[H])=O)Cl
|
| InChi Key |
JMSUDQYHPSNBSN-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C22H21Cl2N3O5/c23-16-9-14(10-17(24)12-16)13-31-22(30)27-7-5-26(6-8-27)4-3-19(28)15-1-2-18-20(11-15)32-21(29)25-18/h1-2,9-12H,3-8,13H2,(H,25,29)
|
| 化学名 |
3,5-dichlorobenzyl 4-(3-oxo-3-(2-oxo-2,3-dihydrobenzo[d]oxazol-6-yl)propyl)piperazine-1-carboxylate
|
| 别名 |
PF-8380; PF 8380; PF8380; 1144035-53-9; 3,5-Dichlorobenzyl 4-(3-oxo-3-(2-oxo-2,3-dihydrobenzo[d]oxazol-6-yl)propyl)piperazine-1-carboxylate; (3,5-Dichlorophenyl)methyl 4-[3-oxo-3-(2-oxo-2,3-dihydro-1,3-benzoxazol-6-yl)propyl]piperazine-1-carboxylate; T582DIM5A4; 1-Piperazinecarboxylic acid, 4-[3-(2,3-dihydro-2-oxo-6-benzoxazolyl)-3-oxopropyl]-, (3,5-dichlorophenyl)methyl ester; UNII-T582DIM5A4;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~209.06 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 0.67 mg/mL (1.40 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 6.7 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 0.67 mg/mL (1.40 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 6.7mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 0.67 mg/mL (1.40 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10 mg/mL (20.91 mM) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0906 mL | 10.4530 mL | 20.9061 mL | |
| 5 mM | 0.4181 mL | 2.0906 mL | 4.1812 mL | |
| 10 mM | 0.2091 mL | 1.0453 mL | 2.0906 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Inhibition of ATX reduces Akt Phosphorylation in GBM cells grown in co-culture.Front Oncol.2013 Sep 17;3:236. |
|---|
 Inhibition of ATX abrogates radiation induced tumor neovascularization.Front Oncol.2013 Sep 17;3:236. |
 Inhibition of ATX in combination with irradiation delays tumor growth in a heterotopic tumor model of GL261.Front Oncol.2013 Sep 17;3:236. |