| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| Other Sizes |
|

| 靶点 |
Glucocorticoid receptor
|
|---|---|
| 体外研究 (In Vitro) |
对糖皮质激素的耐药性或敏感性被认为对儿童急性淋巴细胞白血病的疾病预后至关重要。泼尼松龙对耐药CCRF-CEM白血病细胞系具有延迟的双相作用,低剂量时坏死,高剂量时凋亡。在低剂量下,尽管泼尼松龙诱导了总细胞死亡,但它仍具有显性促有丝分裂作用,而在高剂量下,泼尼松隆的促有丝分化和细胞死亡作用得到了平衡。早期基因微阵列分析显示,40个基因存在显著差异。泼尼松龙的促有丝分裂/双相作用在耐药白血病细胞的情况下具有临床意义。这种方法可能会导致识别未来与糖皮质激素联合治疗中分子药物靶点的候选基因,以及糖皮质激素耐药性的早期标志物[1]。
泼尼松龙(0.002-10 μg/mL;3 天)抑制人白细胞有丝分裂[5]。 |
| 体内研究 (In Vivo) |
一氧化氮被认为参与非特异性细胞免疫。革兰氏阴性细菌内毒素通过诱导一氧化氮合酶II(NOS II)增加吞噬细胞中活性氮中间体(RNI)的产生。抗炎糖皮质激素可减弱内毒素诱导的RNI增加。本研究评估了体内给予泼尼松龙对大肠杆菌脂多糖内毒素(LPS)诱导的大鼠血浆RNI和中性粒细胞NOS II mRNA增加以及RNI产生的影响。我们发现,静脉注射0.5mg/kg亚致死剂量的LPS后2小时内,LPS迅速诱导大鼠中性粒细胞中NOS II的mRNA和RNI(NO2-和NO3-阴离子)的产生。在LPS前15分钟给予药物剂量的泼尼松龙(50微克/kg,im),可减弱中性粒细胞产生NO2-和NO_3-,并抑制LPS刺激的NOS II mRNA。3-氨基-1,2,4-三嗪抑制NO2-和NO3-的产生,而不影响NOS II的基因表达。这些数据表明,LPS迅速诱导NOS II的功能基因表达,泼尼松龙通过抑制其mRNA的转录来阻止NOS II活性的诱导[2]。
短期服用大剂量皮质类固醇后,膈肌萎缩和无力。在本研究中,研究了长期服用中等剂量的氟化和非氟化类固醇对大鼠膈肌收缩特性和组织病理学的影响。60只大鼠每天接受生理盐水、1.0 mg/kg曲安奈德或1.25或5 mg/kg泼尼松龙肌肉注射,持续4周。对照组和两个泼尼松龙组的呼吸和外周肌肉质量同样增加,而曲安奈德会导致严重的肌肉萎缩。对照组的最大强直张力平均为2.23+/-0.54kg/cm2(SD)。5-mg/kg泼尼松龙组膈肌束数量的增加产生了最大强直张力<2.0 kg/cm2(P<0.05)。此外,该组在力量频率方案期间的疲劳性最为明显(P<0.05)。相比之下,曲安奈德导致半松弛时间延长和力-频率曲线向左偏移(P<0.05)。对照组和1.25mg/kg泼尼松龙组的膈肌组织学检查显示正常模式。然而,在5-mg/kg泼尼松龙组中发现了肌源性变化,在曲安奈德组中更为明显。在后一组中发现了选择性IIb型纤维萎缩,但在泼尼松龙组中没有发现。总之,曲安奈德诱导IIb型纤维萎缩,导致呼吸肌力量减弱和力频曲线向左偏移。相比之下,5 mg/kg泼尼松龙引起膈肌收缩特性的改变和组织学变化,而没有纤维萎缩[3]。 由于NZB/NZW小鼠会发展出类似于人类系统性红斑狼疮的免疫性肾炎,因此在这些小鼠中设计了一项研究,以比较三种免疫抑制药物方案的临床和免疫效果。72周内,每组20只小鼠每天接受a)无药物、b)硫唑嘌呤、c)泼尼松龙或d)联合硫唑嘌呤泼尼松隆的口服治疗。联合方案在预防肾脏疾病死亡方面优于单独使用任何一种药物。单独使用泼尼松龙也能显著延长寿命,但不如联合治疗有效。单独使用硫唑嘌呤无效。所有药物均能很好地抑制对外源性抗原(Vi多糖)的抗体反应。所有药物方案均未阻止肾小球中蛋白尿、抗核抗体、Coombs抗体或γ-球蛋白沉积的出现。然而,治疗方案抑制天然DNA抗体的能力与其抑制肾脏疾病的能力密切相关。73只动物尸检中未发现恶性肿瘤,但接受泼尼松龙治疗的组出现了严重的肝损伤。因此,联合治疗优于单独使用任何一种药物,临床上最重要的免疫抑制作用似乎是预防抗DNA抗体形成的能力[4]。 |
| 细胞实验 |
泼尼松龙治疗[1]
根据1个月至12岁儿童静脉注射的平均体内剂量选择泼尼松龙的浓度(详细信息见补充数据,文件:CCRFCEM细胞毒性测定.xls)。此外,皮质醇当量的生物活性估计在40-200nM的范围内。为确保研究涵盖这些范围,将泼尼松龙稀释至以下12个浓度:对照组、10 nM、100 nM、1μM、5.5μM、11μM、22μM、44μM、88μM、175μM、350μM和700μM。 细胞增殖测定 使用NIHON KOHDEN CellTaq-α血液分析仪测定细胞群计数。在开始接触Prednisolone/泼尼松龙后-24小时以及0小时、4小时、24小时、48小时和72小时对细胞进行计数。为此,从每个烧瓶中获得200μl细胞悬浮液,并直接用分析仪计数。 蛋白质提取和蛋白质印迹[1] 在暴露于不同浓度的Prednisolone/泼尼松龙1小时和4小时后收获细胞。如前所述进行蛋白质提取和蛋白质印迹。以牛血清白蛋白为标准,采用Bradford法测定总蛋白含量。通过SDS-PAGE分离蛋白质,并用抗p65抗体进行蛋白质印迹 微阵列分析[1] cDNA微阵列芯片(1200个基因)从TAKARA(人癌症芯片v.40)获得。按照制造商的描述,使用CyScribe Post Labeling试剂盒进行杂交,使用Cy3和Cy5荧光染料。载玻片用微阵列扫描仪扫描。使用ScanArray微阵列采集软件生成图像。使用来自三个实验装置的cDNA,每个装置由三个独立的实验组成。实验装置由以下三对组成:对照组与10 nMPrednisolone(指定为0vs1),10 nM泼尼松龙与700μM泼尼松隆(指定为1vs3),对照组与700μM泼尼松松(指定为0vs3)。如前所述,这是一种“简单循环”实验设计,考虑了样本之间的所有可能组合。原始微阵列数据可作为补充数据。 实时逆转录聚合酶链式反应(qRT-PCR)[1] 使用一步法Plexor™qRT-PCR试剂盒,在4小时和48小时的处理下,从3个样品对照中检测GRIM19(NDUFA13)基因,即10 nM和700μM的Prednisolone。该套引物是使用Promega的在线工具Plexor™引物设计系统v1.2设计的 |
| 动物实验 |
Study design, animals, and treatment [3]
60 adult male Wistar rats, aged 14 wk, weighing 350-400 g, were randomized in quadruplets, into one of four treatment groups: control (C), saline, 0.05 ml/d i.m.; low dose prednisolone (LD), 1.25 mg/kg per d i.m.; high dose prednisolone (HD), 5 mg/kg per d i.m.; or triamcinolone-diacetate (TR), 1 mg/kg per d i.m. Dilution of medication was performed such that with each injection all animals received a similar volume (0.05 ml). During 4 wk the animals were injected daily in the left hindlimb. They were fed ad libitum and weighed twice weekly. After the treatment period, contractile properties, histological, and histochemical characteristics of the diaphragm were examined.[3] Animal/Disease Models: NZB/NZW mice, immune nephritis model[4] Doses: 5 mg/ kg/day Route of Administration: po (oral gavage) 6 days a week for 72 weeks Experimental Results: Dramatically lowered mortality rate and prolonged life Dramatically. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Oral prednisolone reaches a Cmax of 113-1343ng/mL with a Tmax of 1.0-2.6 hours. Oral prednisolone is approximately 70% bioavailable. Prednisolone is over 98% eliminated in urine. A 0.15mg/kg dose of prednisolone has a volume of distribution of 29.3L, while a 0.30mg/kg dose has a volume of distribution of 44.2L. A 0.15mg/kg dose of prednisolone has a clearance of 0.09L/kg/h, while a 0.30mg/kg dose has a clearance of 0.12L/kg/h. A randomized crossover study was conducted to compare the pharmacokinetics and pharmacodynamics of 30 mg prednisolone in a plain oral tablet (Precortisyl) with those of an enteric coated tablet (Deltacortril) in 8 patients (ages 63-81 yr) with chronic obstructive pulmonary disease and in 8 healthy males (ages 22-44 yr). Although drug absorption was considerably slower from the enteric coated tablet, peak plasma levels and total area under the concn-time curve were equivalent for the formulations. Adrenal suppression was significantly less in volunteers after enteric coated than after plain tablets. This difference was not significant in patients. Plasma cortisol levels declined more slowly after enteric coated tablets in both groups. Blood glucose levels increased over 8 hr in both groups. It was concluded that in patients with chronic obstructive pulmonary disease, peak plasma levels and total area under the concn-time curve of plain and enteric coated prednisolone tablets are equivalent; enteric coated tablets result in a lag in the decline of plasma cortisol and, in volunteers, a less marked suppression of cortisol. The transfer of prednisolone to breast milk was studied in 3 nursing women (ages 28-37 yr) who received an intravenous injection of 50 mg prednisolone sodium phosphate (Hydeltrasol). Concn of prednisolone in milk declined more rapidly than in serum, but were similar to expected unbound serum levels. Milk levels ranged from about 15% to 40% of serum levels. The exchange between unbound drug in serum and breast milk appeared to be relatively rapid and bidirectional. An average of 0.025% (0.01-0.49%) of the prednisolone dose was recovered in milk. It was concluded that the transfer of prednisolone to breast milk does not appear to pose a clinically significant risk. The pharmacokinetics of prednisolone after oral and intravenous administration of 10 and 20 mg have been studied. Serum protein binding of prednisolone was also measured after the iv injections. The bioavailability after oral administration was 84.5% after 10 mg and 77.6% after 20 mg (p>0.05). Dose dependent pharmacokinetics were found, the VDss and Clt being significantly larger (p<0.01) after 20 mg iv than after 10 mg iv. The protein binding of prednisolone in all subjects was non-linear, and is the most likely cause of the dose dependent pharmacokinetics, as there was no dose dependent variation in elimination half-time. Doses of 16, 32, 48 and 64 mg prednisolone were administered intravenously to normal volunteers who also received 100 prednisolone orally. Plasma prednisolone concentrations were estimated by quantitative thin layer chromatography. The bioavailability fraction was 1.063 +/- 0.154 (s.d.) indicating complete availability of prednisolone following oral administration. The mean T 1/2 over all doses were 4.11 +/- 0.97 (s.d.) hr and there was no evidence of a dose-related change in its value. The mean systemic clearance over all doses was 0.104 +/- 0.034 (s.d) L/hr/kg. There was no evidence of a dose-related change in clearance or in the apparent volume of distribution (overall mean 0.588 +/- 0.152 L/kg). The area under the plasma concentration-time curve was linearly related to dose. Plasma concentration-time curves normalised for dose were superimposable. It was concluded that over the dose range investigated, non-linear pharmacokinetic behavior had not been demonstrated in this group of normal volunteers. For more Absorption, Distribution and Excretion (Complete) data for PREDNISOLONE (13 total), please visit the HSDB record page. Metabolism / Metabolites Prednisolone can be reversibly metabolized to [prednisone] which is then metabolized to 17α,21-dihydroxy-pregnan-1,4,6-trien-3,11,30-trione (M-XVII), 20α-dihydro-prednisone (M-V), 6βhydroxy-prednisone (M-XII), 6α-hydroxy-prednisone (M-XIII), or 20β-dihydro-prednisone (M-IV). 20β-dihydro-prednisone is metabolized to 17α,20ξ,21-trihydroxy-5ξ-pregn-1-en-3,11-dione(M-XVIII). Prednisolone is metabolized to Δ6-prednisolone (M-XI), 20α-dihydro-prednisolone (M-III), 20β-dihydro-prednisolone (M-II), 6αhydroxy-prednisolone (M-VII), or 6βhydroxy-prednisolone(M-VI). 6αhydroxy-prednisolone is metabolized to 6α,11β,17α,20β,21-pentahydroxypregnan-1,4-diene-3-one (M-X). 6βhydroxy-prednisolone is metabolized to 6β,11β,17α,20β,21-pentahydroxypregnan-1,4-diene-3-one (M-VIII), 6β,11β,17α,20α,21-pentahydroxypregnan-1,4-diene-3-one (M-IX), and 6β,11β,17α,21-tetrahydroxy-5ξ-pregn-1-en-3,20-dione (M-XIV). MVIII is metabolized to 6β,11β,17α,20β,21-pentahydroxy-5ξ-pregn-1-en-3-one (M-XV) and then to MXIV, while MIX is metabolized to 6β,11β,17α,20α,21-pentahydroxy-5ξ-pregn-1-en-3-one (M-XVI) and then to MXIV. These metabolites and their glucuronide conjugates are excreted predominantly in the urine. Reduction of the 4,5 double bond can occur at both hepatic and extrahepatic sites and yields an inactive substance. Subsequent reduction of the 3-ketone substituent to a 3-hydroxyl to form tetrahydrocortisol has been demonstrated only in liver. Most of the ring a - reduced metabolites are enzymatically coupled through the 3-hydroxyl with sulfate or with glucuronic acid to form water soluble sulfate esters or glucuronides, and they are excreted as such. Conjugated mostly in liver but also in kidney. /Human, oral/ In the present study the metabolism of prednisolone in the isolated, perfused, dual recirculating human placental lobule was reexamined, using a perfusate based on tissue culture medium 199. Four metabolites were identified in both the maternal and fetal compartments in 6 hr perfusions by comparison of relative retention times measured by HPLC and capillary GC and of mass spectra recorded by capillary GC/MS with those of authentic reference standards. The steroids were derivatized as the MO-TMS ethers for mass spectral measurements. Analysis of samples from five perfusion experiments resulted in the following percentage conversions after 6 hr perfusion (mean + or - standard deviation, maternal and fetal perfusate, respectively): prednisone (49.1 + or - 7.8, 49.1 + or - 6.6), 20 alpha-dihydroprednisone (0.84 + or - 0.29, 0.81 + or - 0.35), 20 beta-dihydroprednisone (39.1 + or - 6.7, 39.2 + or - 5.9), 20 beta-dihydroprednisolone (6.8 + or - 2.7, 6.3 + or - 1.6) and unmetabolized prednisolone (4.1 + or - 1.8, 4.6 + or - 2.1). No evidence was found for metabolites formed by 6 beta-hydroxylation or cleavage of the C17-C20 bond. A randomized, four-way cross-over study was conducted in eight healthy male volunteers to determine the relative and absolute bioavailability of prednisone (PN) and prednisolone (PL). PN and PL were administered as single, oral 10-mg tablet doses and as 10-mg zero-order 0.5-hour intravenous infusions. Comparable mean PN and PL maximum plasma concentrations (Cmax), times for Cmax, areas under the plasma concentration-time curves (AUC), and apparent elimination rate constants between tablet treatments demonstrated that PN and PL tablets were bioequivalent. Absolute bioavailability (F) determinations based on plasma PL concentrations were independent of which IV treatment was used as reference and indicated complete systemic availability of PL from both PN and PL tablets. However, F based on plasma PN data was contradictory. Using IV PN as reference, approximately 70% systemic availability was observed from both tablets, whereas using IV PL as reference, systemic availability was greater than unity. PN and PL are model compounds that exemplify the difficulties involved in accurately determining the relative and absolute bioavailability of substances that undergo reversible metabolism. Prednisone, prednisolone, and methylprednisolone are currently administered in association with cyclosporin A in the postoperative treatment of transplant patients. The aim of this work was to evaluate the effects of these corticosteroids on the expression of several forms of cytochromes p450, including p450 1A2, 2D6, 2E1, and 3A, and on cyclosporin A oxidase activity in human liver. For this purpose, human hepatocytes prepared from lobectomies were maintained in culture in a serum-free medium, in collagen-coated dishes, for 96-144 hr, in the absence or presence of 50-100 uM corticosteroids, rifampicin, or dexamethasone. To mimic more closely the current clinical protocol, hepatocyte cultures were also co-treated with corticosteroids and cyclosporin A or ketoconazole (a selective inhibitor of cytochromes p450 3A). Cyclosporin A oxidase activity, intracellular retention of cyclosporin A oxidized metabolites within hepatocytes, accumulation of cytochromes p450 proteins and corresponding messages, and de novo synthesis and half-lives of these cytochromes p450 were measured in parallel in these cultures. Our results, obtained from seven different hepatocyte cultures, showed that 1) dexamethasone and prednisone, but not prednisolone or methylprednisolone, were inducers of cytochrome p450 3A, at the level of protein and mRNA accumulation, as well as of cyclosporin A oxidase activity, known to be predominantly catalyzed by these cytochromes p450; 2) although corticosteroids are known to be metabolized in human liver, notably by cytochrome p450 3A, partial or total inhibition of this cytochromes p450 by cyclosporin or ketoconazole, respectively, did not affect the inducing efficiency of these molecules; 3) corticosteroids did not affect the half-life of cytochrome p450 3A or the accumulation of other forms of cytochromes p450, including 1A2, 2D6, and 2E1; 4) chronic treatment of cells with cyclosporin did not affect cytochrome p450 3A accumulation; 5) corticosteroids were all competitive inhibitors of cyclosporin A oxidase in human liver microsomes, with Ki values of 61 + or - 12, 125 + or - 25, 190 + or - 38, and 210 + or - 42 uM for dexamethasone, prednisolone, prednisone, and methylprednisolone, respectively; and 6) chronic treatment of cells with corticosteroids did not influence the excretion of oxidized metabolites of cyclosporin from the cells. Biological Half-Life Prednisolone has a plasma half life of 2.1-3.5 hours. This half life is shorter in children and longer in those with liver disease. ...Prednisolone (60 mg/sq m/day in three divided doses) was administered both orally and intravenously /to 23 children with acute lymphoblastic leukemia (ALL) (aged 2-15 years)/, and samples were obtained on several days during the initial 5 weeks of remission induction therapy. ...The median unbound clearance (32 L/hr/sq m) was lower, and the half-life (3.6 hr) longer than previously reported in childhood ALL. Doses of 16, 32, 48 and 64 mg prednisolone were administered intravenously to normal volunteers who also received 100 prednisolone orally. ...The mean T 1/2 over all doses were 4.11 +/- 0.97 (s.d.) hr and there was no evidence of a dose-related change in its value. |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
Seizures have been observed in patients receiving cyclosporine and high doses of methylprednisolone. /Methylprednisolone In one study, women taking oral contraceptives or postmenopausal estrogen therapy were given prednisolone concurrently. Alterations in metabolism of prednisolone, including incr half-life, were consistent with a potential for enhanced pharmacologic effect or toxicity when prednisolone was added to an estrogen regimen. Ketoconazole inhibits the deposition of ... prednisolone by inhibiting 6beta-hydroxylase, thereby prolonging the adrenal suppressive effect of ... /prednisolone/. Drugs reported to increase blood levels of cyclosporine include ... prednisolone. For more Interactions (Complete) data for PREDNISOLONE (26 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse ip > 1000 mg/kg body weight /Prednisolone acetate/ LD Mouse ip 767 mg/kg body weight LD50 Swiss mouse oral 1680 mg/kg body weight LD50 Sherman rat (male) sc 147 mg/kg body weight Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Amounts of prednisolone in breastmilk are very low. No adverse effect have been reported in breastfed infants with maternal use of any corticosteroid during breastfeeding. Although it is often recommended to avoid breastfeeding for 4 hours after a dose this maneuver is not necessary because prednisolone milk levels are very low. Medium to large doses of corticosteroids given systemically or injected into joints or the breast have been reported to cause temporary reduction of lactation. Because absorption from the eye is limited, ophthalmic prednisolone would not be expected to cause any adverse effects in breastfed infants. To substantially diminish the amount of drug that reaches the breastmilk after using eye drops, place pressure over the tear duct by the corner of the eye for 1 minute or more, then remove the excess solution with an absorbent tissue. ◉ Effects in Breastfed Infants None reported with prednisolone or any other corticosteroid. In a prospective follow-up study, 6 nursing mothers reported taking prednisone (dosage unspecified) with no adverse infant effects. There are several reports of mothers breastfeeding during long-term use of corticosteroids with no adverse infant effects: prednisone 10 mg daily (2 infants) and prednisolone 5 to 7.5 mg daily (14 infants). A woman who was nursing (extent not stated) her newborn infant was treated for pemphigus with oral prednisolone 25 mg daily, with the dosage increased over 2 weeks to 60 mg daily. She was also taking cetirizine 10 mg daily and topical betamethasone 0.1% twice daily to the lesions. Because of a poor response, the betamethasone was changed to clobetasol propionate ointment 0.05%. She continued breastfeeding throughout treatment and her infant was developing normally at 8 weeks of age and beyond. A woman with pemphigoid gestationis was treated with prednisolone in a dosage tapering from 0.7 mg/kg daily to 1 mg daily during breastfeeding. She also received courses of intravenous immune globulin 2 grams/kg over 3 days at 4, 9 and 13 weeks postpartum. She breastfed her infant (extent not stated) for 3 months with no problems noted. Two mothers with systemic lupus erythematosus were reported who took prednisolone 30 or 40 mg daily during pregnancy and lactation as well as tacrolimus 3 mg daily. Three years after birth, both children were healthy. The durations of lactation were not stated. A woman with rheumatoid arthritis refractory to etanercept took sarilumab 200 mg every two weeks during pregnancy until 37 weeks of gestation. She was also taking prednisolone 10 mg and tacrolimus 3 mg daily. She delivered a healthy infant at 38 weeks of gestation and breastfed her infant. Prednisolone was continued postpartum, tacrolimus was restarted at 7 days postpartum, and sarilumab was restarted at 28 days postpartum. The mother continued to breastfeed until 6 months postpartum. The infant was vaccinated with multiple live vaccines after reaching six months old, including the Bacille-Calmette-Guerin vaccine, with no adverse effects. ◉ Effects on Lactation and Breastmilk Published information on the effects of prednisolone on serum prolactin or on lactation in nursing mothers was not found as of the revision date. Medium to large doses of corticosteroids given systemically or injected into joints or the breast have been reported to cause temporary reduction of lactation. A study of 46 women who delivered an infant before 34 weeks of gestation found that a course of another corticosteroid (betamethasone, 2 intramuscular injections of 11.4 mg of betamethasone 24 hours apart) given between 3 and 9 days before delivery resulted in delayed lactogenesis II and lower average milk volumes during the 10 days after delivery. Milk volume was not affected if the infant was delivered less than 3 days or more than 10 days after the mother received the corticosteroid. An equivalent dosage regimen of prednisolone might have the same effect. A study of 87 pregnant women found that betamethasone given as above during pregnancy caused a premature stimulation of lactose secretion during pregnancy. Although the increase was statistically significant, the clinical importance appears to be minimal. An equivalent dosage regimen of prednisolone might have the same effect. 5755 man TDLo oral 9 mg/kg/2W-I BEHAVIORAL: TOXIC PSYCHOSIS Drug Intelligence and Clinical Pharmacy., 18(603), 1984 [PMID:6745088] 5755 women TDLo oral 14 mg/kg/13D-I BEHAVIORAL: TOXIC PSYCHOSIS Drug Intelligence and Clinical Pharmacy., 18(603), 1984 [PMID:6745088] 5755 rat LD50 intraperitoneal 2 gm/kg Advances in Teratology., 3(181), 1968 5755 rat LD50 subcutaneous 147 mg/kg Toxicology and Applied Pharmacology., 8(250), 1966 [PMID:5956877] 5755 rat LD50 intravenous 120 mg/kg Pharmaceutical Chemistry Journal, 16(63), 1982 Protein Binding Prednisolone's protein binding is highly variable, ranging from 65-91% in healthy patients. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Anti-Inflammatory Agents, Steroidal; Antineoplastic Agents, Hormonal; Glucocorticoids, Synthetic Ophthalmic corticosteroids are indicated in the treatment of corticosteroid-responsive allergic and inflammatory conditions of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the globe. /Corticosteroids (Ophthalmic); Included in US product labeling/ VET: Hormonal therapy for neoplasia commonly involves the use of glucocorticoids. Direct antitumor effects are related to their lympholytic properties; glucocorticoids can inhibit mitosis, RNA synthesis, and protein synthesis in sensitive lymphocytes. Glucocorticoids are considered cell-cycle nonspecific and are often used in chemotherapeutic protocols after induction by another agent. Prednisolone /is/ commonly used to treat lymphoreticular neoplasms in combination with other drugs. Because /it/ readily enters the CSF, ... prednisolone /is/ especially useful in treatment of leukemias and lymphomas of the CNS. Indicated in a wide range of endocrine, rheumatic, allergic, dermatologic, respiratory, hematologic, neoplastic, and other disorders. For more Therapeutic Uses (Complete) data for PREDNISOLONE (28 total), please visit the HSDB record page. Drug Warnings VET: IT OFTEN MAY BE CONTRAINDICATED IN CONGESTIVE HEART FAILURE, DIABETES OR OSTEOPOROSIS. EXCEPT FOR EMERGENCY LIFE SAVING USE, IT SHOULD BE OMITTED IN TUBERCULOSIS, CHRONIC NEPHRITIS, CUSHINGOID SYNDROMES, & PEPTIC ULCER CASES. Side effects and compliance were examined in 63 pediatric patients (ages 10 mo-14 yr) with acute asthma who received an oral dose of 1-2 mg/kg prednisolone (Solone; Panafcortelone) as a whole or crushed tablet or in liquid form for 7 days. Up to 44% of patients either refused to take or vomited the drug on the first day. Improved acceptability of prednisolone occurred with time, but prescribing practices indicated short-term treatment of 1 to 4 days was common. Abdominal pain and mood changes occurred in 19% and 80% of patients, respectively, at some stage of the study period. It was concluded that oral prednisolone is poorly tolerated in pediatric patients and its use may lead to suboptimal therapy. Glucocorticoid use in children is not only associated with the side effects which are seen in adults, but also with severe adverse effects on statural growth. As little as 2.5-5.0 mg prednisolone/day can cause a retardation in statural growth. A direct relationship exists between the dose of glucocorticoid used and statural growth. The use of knemometry, a sensitive technique for measuring the growth of long bones in children has increased the accuracy of growth rate measurements. Many factors, such as disease process, sex, daily vs alternate day therapy, ethnic variations or whether the patient has been immobilized must be considered when evaluating the effects on stature of a particular glucocorticoid. RESULTS FROM CONTROLLED TRIAL, INDICATE THAT PREDNISOLONE TREATMENT IS NOT BENEFICIAL & CAN BE DETRIMENTAL IN ACUTE NEUROPATHY OF UNDETERMINED ETIOLOGY. For more Drug Warnings (Complete) data for PREDNISOLONE (48 total), please visit the HSDB record page. Pharmacodynamics Corticosteroids bind to the glucocorticoid receptor, inhibiting pro-inflammatory signals, and promoting anti-inflammatory signals. Prednisolone has a short duration of action as the half life is 2.1-3.5 hours. Corticosteroids have a wide therapeutic window as patients make require doses that are multiples of what the body naturally produces. Patients taking corticosteroids should be counselled regarding the risk of hypothalamic-pituitary-adrenal axis suppression and increased susceptibility to infections. |
| 分子式 |
C21H28O5
|
|
|---|---|---|
| 分子量 |
360.44
|
|
| 精确质量 |
360.193
|
|
| 元素分析 |
C, 69.98; H, 7.83; O, 22.19
|
|
| CAS号 |
50-24-8
|
|
| 相关CAS号 |
Prednisolone;50-24-8; Prednisolone;50-24-8;Prednisolone hemisuccinate;2920-86-7;Prednisolone acetate;52-21-1; 630-67-1 (sodium metazoate); 72064-79-0 (valerate acetate); 125-02-0 (Na+ phosphate)
|
|
| PubChem CID |
5755
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
570.6±50.0 °C at 760 mmHg
|
|
| 熔点 |
240 °C (dec.)(lit.)
|
|
| 闪点 |
313.0±26.6 °C
|
|
| 蒸汽压 |
0.0±3.6 mmHg at 25°C
|
|
| 折射率 |
1.612
|
|
| LogP |
1.5
|
|
| tPSA |
94.83
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
26
|
|
| 分子复杂度/Complexity |
724
|
|
| 定义原子立体中心数目 |
7
|
|
| SMILES |
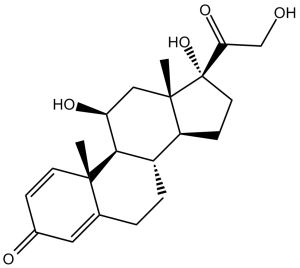
C[C@]12C[C@@H]([C@H]3[C@H]([C@@H]1CC[C@@]2(C(=O)CO)O)CCC4=CC(=O)C=C[C@]34C)O
|
|
| InChi Key |
OIGNJSKKLXVSLS-VWUMJDOOSA-N
|
|
| InChi Code |
InChI=1S/C21H28O5/c1-19-7-5-13(23)9-12(19)3-4-14-15-6-8-21(26,17(25)11-22)20(15,2)10-16(24)18(14)19/h5,7,9,14-16,18,22,24,26H,3-4,6,8,10-11H2,1-2H3/t14-,15-,16-,18+,19-,20-,21-/m0/s1
|
|
| 化学名 |
(8S,9S,10R,11S,13S,14S,17R)-11,17-Dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-3-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.77 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.77 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (5.77 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7744 mL | 13.8719 mL | 27.7439 mL | |
| 5 mM | 0.5549 mL | 2.7744 mL | 5.5488 mL | |
| 10 mM | 0.2774 mL | 1.3872 mL | 2.7744 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Study to Test Whether Spesolimab Helps People With a Skin Condition Called Pyoderma Gangrenosum
CTID: NCT06624670
Phase: Phase 3 Status: Not yet recruiting
Date: 2024-11-19