| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
| 靶点 |
Bromodomain (BRD)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
Birabresib (OTX-015) (500 nM) 暴露会导致 BRD2、BRD4 和 c-MYC 显着减少,HEXIM1 蛋白增加,而 BRD3 表达未改变。然而,c-MYC、BRD2、BRD3、BRD4 和 HEXIM1 mRNA 水平确实与暴露于 Birabresib (OTX-015) 后的活力相关[2]。 Birabresib (OTX-015) (0.1, 1, 5 μM) 给药可促进接受抑制性抗逆转录病毒治疗 (ART) 的感染患者的静息 CD4+ T 细胞中的 HIV-1 全长转录物和病毒生长,同时对 T 细胞产生低毒性和影响细胞激活。 Birabresib 介导的 HIV-1 激活涉及 CDK9 占用率和 RNAP II C 末端结构域 (CTD) 磷酸化的增加[3]。
Birabresib(OTX-015)是BRD2、BRD3和BRD4的强效抑制剂,EC50值为10至19 nM。以浓度依赖的方式添加OTX015可抑制OTX015与BRD2、BRD3和BRD4的结合,表明存在竞争性抑制。OTX015抑制了BRD2、BRD3和BRD4与AcH4的结合,IC50值为92至112 nM。OTX015抑制多种人癌症细胞系的生长;对于大多数测试的血液系统恶性肿瘤,GI50值范围为60至200 nM。[1] 暴露于Birabresib(OTX-015)会导致急性白血病细胞系和患者来源的白血病细胞在亚摩尔浓度下出现细胞生长抑制、细胞周期阻滞和凋亡,如典型的JQ1 BET抑制剂所述。JQ1和OTX15治疗在敏感细胞系中诱导了相似的基因表达谱,包括c-MYC减少和HEXIM1增加。OTX015暴露还诱导了BRD2、BRD4和c-MYC的显著降低以及HEXIM1蛋白的增加,而BRD3的表达没有变化。然而,c-MYC、BRD2、BRD3、BRD4和HEXIM1 mRNA水平与暴露于OTX015后的存活率无关。OTX015与其他表观遗传修饰药物帕诺司他和阿扎胞苷的顺序组合对KASUMI细胞系的生长具有协同作用。我们的结果表明,OTX015和JQ1在白血病细胞中具有相似的生物学效应,支持在复发/难治性白血病患者的Ib期试验中对OTX015进行评估。[2] Birabresib(OTX-015)在三种人TNBC衍生细胞系HCC1937、MDA-MB-231和MDA-MB-468中进行了检测,72小时后均显示出抗增殖活性(GI50=75-650nM)。这伴随着细胞周期阻滞和癌症干细胞标志物的表达降低。然而,c-MYC蛋白和mRNA水平仅在MDA-MB-468细胞中下调。基因集富集分析显示,参与转录、染色质和细胞周期表观遗传控制的基因受到调控,而与干性相关的基因则受到下调。在体外,与依维莫司的组合在HCC1937和MDA-MB-231细胞中是相加的,但在MDA-MB-468细胞中是拮抗的[4]。 |
||
| 体内研究 (In Vivo) |
与给予赋形剂的小鼠相比,Birabresib (OTX-015) (50 mg/kg) 显着 (p<0.05) 降低了 MDA-MB-231 小鼠异种移植物的肿瘤负荷。 Birabresib (OTX-015) 与 2 mg/kg RAD001 联合使用比单独使用 Birabresib 更有效[4]。
MDA-MB-231异种移植物的体内实验[4] 基于体外联合研究,选择MDA-MB-231细胞系生成小鼠异种移植物,以评估单独使用和与依维莫司联合使用比拉布雷西布(OTX-015)的体内效果。与对照组相比,OTX015治疗的小鼠在治疗开始后7天肿瘤质量显著减少(p<0.05)(图5B)。第23天记录的最佳T/C值为41.3%(表2)。另一方面,与对照组相比,单独使用依维莫司对肿瘤生长没有实质性影响。 在这种实验环境中,Birabresib(OTX-015)/依维莫司组合是最有效的方法,从治疗开始后第4天开始,与赋形剂治疗的动物相比,肿瘤质量显著减少(p<0.05)(图5B),第23天的最佳T/C为20.7%(表2)。此外,在整个治疗期间,从第7天和第21天开始,OTX015/依维莫司联合组的平均肿瘤重量分别显著低于依维莫司和OTX015单药组(p<0.05)。更重要的是,治疗结束后,用联合药物治疗的小鼠的肿瘤仍然明显小于用单一药物治疗的动物的肿瘤(p<0.05)(图5B)。值得注意的是,依维莫司治疗组的所有小鼠都有明显的毒性迹象,如平均体重下降所示(图5C)。因此,从治疗开始后的第14天开始,将依维莫司方案修改为单药和联合方案的每周一次。 紫杉醇在临床上广泛用于治疗TNBC,在一项单独的实验中进行了评估,提供了一个临床基准(补充图S2)。该药物在第23天显示出最佳T/C为28.9%的活性(表2)。根据三个参数(T/C%、AGD和LCK)比较Birabresib(OTX-015)、依维莫司、OTX015/依维莫司联合用药和紫杉醇的药理学疗效,清楚地表明OTX015/-依维莫司组合是最有效的治疗方法(表2)。 在治疗期结束时,在最后一次给药后4小时处死Birabresib(OTX-015)组的一半动物,以确定血液和组织中的药物水平。肿瘤和血浆中的OTX015浓度相当,明显高于瘤周组织中观察到的水平(p<0.05)(图5D)。这些药物浓度约为2μM(在血浆和肿瘤组织中),表明在肿瘤环境中可以实现体外使用的浓度,这与Gaudio等人最近在淋巴瘤模型中的一份报告是一致的。对于剩余的动物,如上所述,再监测肿瘤和体重20天。 c-MYC、BETs和CSC标志物在MDA-MB-231异种移植物中的表达[4] 评估治疗开始4周后处死的异种移植物小鼠的肿瘤组织中关键基因的变化(图5E)。与载体处理的动物相比,所有实验组均未显示出c-MYC、BRD2/3 mRNA表达的变化。然而,与赋形剂对照组相比,用Birabresib(OTX-015)/依维莫司联合治疗的小鼠BRD4 mRNA水平显著降低(p<0.05)。同样,与体外情况一样,OTX015诱导CD24 mRNA水平显著升高,同时CD44表达降低(p<0.05)。然而,OTX015/依维莫司联合用药仅导致CD24显著增加(p<0.05),而不影响CD44表达。另一方面,依维莫司诱导CD24表达显著降低(p<0.05),对CD44没有影响。OTX015和联合治疗均下调了EpCAM、NANOG和OCT4干性标志物的mRNA水平(p<0.05)。相比之下,依维莫司没有改变EpCAM和OCT4的表达,但显著上调了NANOG mRNA水平(p<0.05)。 |
||
| 酶活实验 |
为了评估OTX015与BRD2、BRD3和BRD4的结合,将表达BRD的CHO细胞裂解物(来自转染了Flag标记的BRD2、BR D3或BRD4表达质粒或单独载体的CHO细胞)、铕偶联的抗Flag抗体、XL-665偶联的链霉抗生物素蛋白和生物素化的OTX015在室温下孵育0.2至2小时。使用EnVision 2103多标签阅读器通过TR-FRET测量荧光,并使用PRISM 5.02版通过非线性回归计算结合的EC50。使用类似的系统,通过孵育生物素偶联的-AcH4、表达BRD的CHO细胞裂解物、铕偶联的抗Flag抗体和XL-665偶联的链霉抗生物素蛋白,评估了OTX015对BRD2、BRD3和BRD4与乙酰化组蛋白H4(AcH4)结合的影响。使用EnVision 2103多标记阅读器通过TR-FRET测量荧光,并通过将不含生物素偶联物dAcH4的样品的值定义为0%,将不含OTX015的样品定义为100%来计算结合百分比。使用PRISM 5.02版通过非线性回归计算IC50值。OTX015对癌症细胞增殖的影响通过在OTX015浓度增加的情况下孵育人肿瘤细胞72小时并使用基于四唑盐(WST-8)的比色测定法评估增殖来评估[1]。
|
||
| 细胞实验 |
MTT法、凋亡评估和细胞周期分析[2]
对于MTT分析,将细胞以每孔1×106的密度接种在24孔板中,用浓度为0.01nM-10μM的Birabresib(OTX-015)处理72小时。将细胞转移到96孔板中并在37°C的黑暗中用0.5mg/mL的3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四唑孵育4小时。然后用25%十二烷基硫酸钠(SDS)裂解缓冲液裂解细胞,使用Promega Microplate Reader在570nm处读取吸光度。对每种细胞系进行三次独立实验,未经处理的细胞用作阴性对照。使用Prism®v6软件计算半最大抑制浓度(IC50)值。 对于细胞周期分析,将1×106个细胞用25-500nM浓度的Birabresib(OTX-015)处理48小时,然后收获,在PBS中洗涤,并在70%冰冷的乙醇中固定。细胞与100μg/mL RNA酶一起孵育,并在37°C下用25μg/mL碘化丙啶(PI;Becton Dickinson)染色30分钟。 为了进行凋亡分析,将1×106个来自患者或细胞系的细胞重新悬浮在1ml培养基中,用Birabresib(OTX-015)处理72小时。使用FACSCalibur流式细胞仪检测凋亡细胞。根据制造商的说明,在室温下用5μg/mL PI和Annexin-V-FITC对细胞染色15分钟。凋亡细胞被定义为有或没有PI摄取的膜联蛋白V+。 MOLT-4/CCR5生长测定[3] 纯化的静息CD4+T细胞(5×106)用PMA加离子霉素或比拉布雷西(OTX-015)处理18小时,并用1ml无菌PBS洗涤以去除残留药物。然后将静止的CD4+T细胞与MOLT-4/CCR5细胞在8ml RPMI1640培养基中培养,并在六孔培养板的单个孔中加入10%FBS。在培养4天和7天后,将孔重新悬浮并以1:2的比例分开,将培养基体积调节至每孔8ml。培养14天后,使用HIV-1 p24抗原ELISA试剂盒评估病毒生长。 细胞活力的测量和T细胞活化标志物和HIV-1受体/共受体的检测[3] 将健康个体的PBMC置于96孔板中,与Birabresib(OTX-015)一起孵育48小时。使用所述的细胞计数试剂盒-8测量细胞存活率72。为了测量细胞活化状态和HIV-1受体/共受体存在的变化,将从健康供体分离的CD4+淋巴细胞与前列素、OTX015或OTX015/前列素一起孵育48小时 h,在4°C下用抗CD25、抗CD69、抗HLA-DR、抗CD4、抗CCR5或抗CXCR4抗体免疫染色20分钟。将细胞固定在1%PFA中,并通过流式细胞术进行分析。 药物组合研究[4] 将细胞以20000个细胞/ml的密度接种在96孔板(100μl/孔)中,24小时后用不同浓度的比拉布雷西(OTX-015)或依维莫司或两种药物的组合处理72小时。然后将细胞与0.8 mg/ml MTT(3-[4,5-二甲基噻唑-2-基]-2,5-二苯基溴化四氮唑)孵育2-4小时。将细胞沉淀重新悬浮在0.05ml DMSO中,使用Infinte 200微孔板读数在560nm处测量吸光度。如前所述,使用Prism 5.00软件确定OTX015和依维莫司EC50值。在至少三个独立实验中进行了三次检测。根据Chou-Talalay算法,使用Compuscyn软件对结果进行了进一步分析。根据计算的组合指数值,<0.90表示协同作用,0.9至≤1.10表示加性作用,>1.10表示拮抗作用。 |
||
| 动物实验 |
|
||
| 参考文献 | |||
| 其他信息 |
Birabresib (OTX-015) is a potent bromodomain (BRD2/3/4) inhibitor.
Birabresib is under investigation in clinical trial NCT02698176 (A Dose Exploration Study With MK-8628 in Participants With Selected Advanced Solid Tumors (MK-8628-006)). Birabresib is a synthetic, small molecule inhibitor of the BET (Bromodomain and Extra-Terminal) family of bromodomain-containing proteins 2, 3 and 4 with potential antineoplastic activity. Upon administration, birabresib binds to the acetylated lysine recognition motifs on the bromodomain of BET proteins, thereby preventing the interaction between the BET proteins and acetylated histone peptides. This disrupts chromatin remodeling and gene expression. Prevention of the expression of certain growth-promoting genes, including c-Myc-dependent target genes, may lead to an inhibition of tumor cell growth. Characterized by a tandem repeat of bromodomain at the N-terminus, the BET proteins BRD2, BRD3, BRD4 are transcriptional regulators that play an important role in cellular growth. Introduction: The BET bromodomain proteins, including BRD2, BRD3, and BRD4, have emerged as major epigenetic regulators of proliferation and differentiation and also have been associated with predisposition to dyslipidemia or improper regulation of adipogenesis, elevated inflammatory profile, and increased susceptibility to autoimmune disease. OTX015, a novel thienotriazolodiazepine compound, was identified in a cell-based screen for inhibitors of cell adhesion. Subsequently, it was evaluated for inhibition of the binding between acetylated histone and BRD2, BRD3, and BRD4 and antiproliferative effects in both in vitro and in vivo tumor models. Material and Methods: To assess binding of OTX015 to BRD2, BRD3, and BRD4, BRD-expressing CHO cell lysate (from CHO cells transfected with expression plasmids for Flag-tagged BRD2, BRD3, or BRD4 or vector alone), europium-conjugated anti-Flag antibody, XL-665-conjugated streptavidin, and biotinylated OTX015 were incubated at room temperature for 0.2 to 2h. Fluorescence was measured by TR-FRET using an EnVision 2103 Multilabel Reader and EC50 for binding was calculated by nonlinear regression using PRISM version 5.02. Using a similar system, the effect of OTX015 on binding of BRD2, BRD3, and BRD4 to acetylated histone H4 (AcH4) was evaluated by incubating biotin-conjugated -AcH4, BRD-expressing CHO cell lysate, europium-conjugated anti-Flag antibody, and XL-665-conjugated streptavidin. Fluorescence was measured by TR-FRET using an EnVision 2103 Multilabel Reader and percent binding was calculated by defining the value of the sample without biotin conjugate dAcH4 as 0% and the sample without OTX015 as 100%. The IC50 value was calculated by nonlinear regression using PRISM version 5.02. Effects of OTX015 on cancer cell proliferation were evaluated by incubating human tumor cells for 72 h with increasing concentrations of OTX015 and assessing proliferation using a tetrazolium salt (WST-8)-based colorimetric assay. To assess antiproliferative effects in vivo, BLAB/c-nu/nu mice bearing established Ty82 BRD-NUT midline carcinoma xenografts were given OTX015 (0, 10, 30 or 100 mg/kg qd or 10 mg/kg bid) by oral gavage over 14 days. Animals were sacrificed on day 15 and tumors were extracted and weighed. Results: OTX015 was a potent inhibitor of BRD2, BRD3, and BRD4, with EC50 values from 10 to 19 nM. Binding of OTX015 to BRD2, BRD3, and BRD4 was inhibited by addition of OTX015 in a concentration-dependent manner, suggesting competitive inhibition. OTX015 inhibited the binding of BRD2, BRD3, and BRD4 to AcH4, with IC50 values from 92 to 112 nM. OTX015 inhibited the growth of a variety of human cancer cell lines; for most hematologic malignancies tested, GI50 values ranged from 60 to 200 nM. Oral administration of OTX015 significantly inhibited the growth of Ty82 BRD-NUT midline carcinoma tumors in nude mice, showing 79% TGI at 100 mg/kg qd and 61% TGI at 10 mg/kg bid. Conclusions: OTX015 is a potent inhibitor of BRD2, BRD3, and BRD4 and inhibits the binding of BRD2, BRD3, and BRD4 to AcH4. OTX015 showed significant anti-tumor activity both in vitro and in vivo tumor models. These findings encouraged the clinical development of OTX015, which is currently in Phase 1 studies in patients with advanced hematologic malignancies (ClinicalTrials.gov Identifier NCT01713582).[1] The bromodomain (BRD) and extraterminal (BET) proteins including BRD2, BRD3 and BRD4 have been identified as key targets for leukemia maintenance. A novel oral inhibitor of BRD2/3/4, the thienotriazolodiazepine compound OTX015, suitable for human use, is available. Here we report its biological effects in AML and ALL cell lines and leukemic samples. Exposure to OTX015 lead to cell growth inhibition, cell cycle arrest and apoptosis at submicromolar concentrations in acute leukemia cell lines and patient-derived leukemic cells, as described with the canonical JQ1 BET inhibitor. Treatment with JQ1 and OTX15 induces similar gene expression profiles in sensitive cell lines, including a c-MYC decrease and an HEXIM1 increase. OTX015 exposure also induced a strong decrease of BRD2, BRD4 and c-MYC and increase of HEXIM1 proteins, while BRD3 expression was unchanged. c-MYC, BRD2, BRD3, BRD4 and HEXIM1 mRNA levels did not correlate however with viability following exposure to OTX015. Sequential combinations of OTX015 with other epigenetic modifying drugs, panobinostat and azacitidine have a synergic effect on growth of the KASUMI cell line. Our results indicate that OTX015 and JQ1 have similar biological effects in leukemic cells, supporting OTX015 evaluation in a Phase Ib trial in relapsed/refractory leukemia patients.[2] None of the currently used anti-HIV-1 agents can effectively eliminate latent HIV-1 reservoirs, which is a major hurdle to a complete cure for AIDS. We report here that a novel oral BET inhibitor OTX015, a thienotriazolodiazepine compound that has entered phase Ib clinical development for advanced hematologic malignancies, can effectively reactivate HIV-1 in different latency models with an EC50 value 1.95–4.34 times lower than JQ1, a known BET inhibitor that can reactivate HIV-1 latency. We also found that OTX015 was more potent when used in combination with prostratin. More importantly, OTX015 treatment induced HIV-1 full-length transcripts and viral outgrowth in resting CD4+ T cells from infected individuals receiving suppressive antiretroviral therapy (ART), while exerting minimal toxicity and effects on T cell activation. Finally, biochemical analysis showed that OTX015-mediated activation of HIV-1 involved an increase in CDK9 occupancy and RNAP II C-terminal domain (CTD) phosphorylation. Our results suggest that the BET inhibitor OTX015 may be a candidate for anti-HIV-1-latency therapies.[3] Triple-negative breast cancer (TNBC) is an aggressive and heterogeneous subgroup of breast tumors clinically defined by the lack of estrogen, progesterone and HER2 receptors, limiting the use of the targeted therapies employed in other breast malignancies. Recent evidence indicates that c-MYC is a key driver of TNBC. The BET-bromodomain inhibitor OTX015 (MK-8628) has potent antiproliferative activity accompanied by c-MYC down-regulation in several tumor types, and has demonstrated synergism with the mTOR inhibitor everolimus in different models. The aim of this study was to evaluate the anti-tumor activity of OTX015 as single agent and in combination with everolimus in TNBC models. OTX015 was assayed in three human TNBC-derived cell lines, HCC1937, MDA-MB-231 and MDA-MB-468, all showing antiproliferative activity after 72 h (GI50 = 75-650 nM). This was accompanied by cell cycle arrest and decreased expression of cancer stem cells markers. However, c-MYC protein and mRNA levels were only down-regulated in MDA-MB-468 cells. Gene set enrichment analysis showed up-regulation of genes involved in epigenetic control of transcription, chromatin and the cell cycle, and down-regulation of stemness-related genes. In vitro, combination with everolimus was additive in HCC1937 and MDA-MB-231 cells, but antagonistic in MDA-MB-468 cells. In MDA-MB-231 murine xenografts, tumor mass was significantly (p < 0.05) reduced by OTX015 with respect to vehicle-treated animals (best T/C = 40.7%). Although everolimus alone was not active, the combination was more effective than OTX015 alone (best T/C = 20.7%). This work supports current clinical trials with OTX015 in TNBC (NCT02259114).[4] |
| 分子式 |
C25H22CLN5O2S
|
|---|---|
| 分子量 |
491.992482662201
|
| 精确质量 |
491.118
|
| 元素分析 |
C, 61.03; H, 4.51; Cl, 7.21; N, 14.23; O, 6.50; S, 6.52
|
| CAS号 |
1983196-25-3
|
| 相关CAS号 |
Birabresib;202590-98-5; 204587-26-8 (dihydrate)
|
| PubChem CID |
118704772
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
4.5
|
| tPSA |
121
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
770
|
| 定义原子立体中心数目 |
1
|
| SMILES |
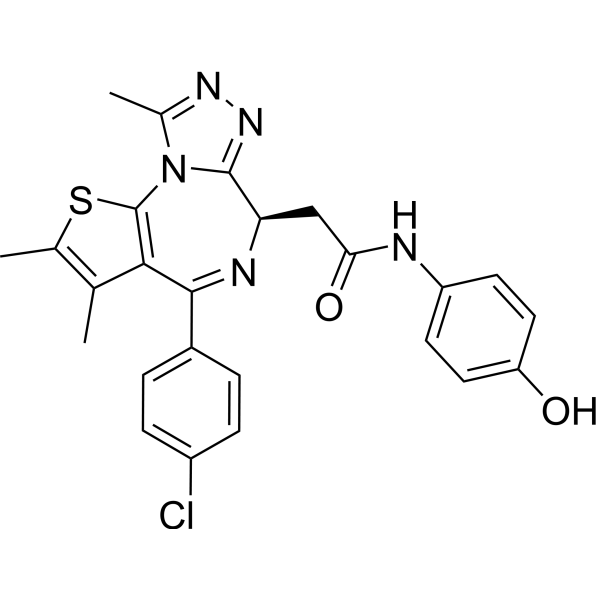
CC1=C(SC2=C1C(=N[C@@H](C3=NN=C(N32)C)CC(=O)NC4=CC=C(C=C4)O)C5=CC=C(C=C5)Cl)C
|
| InChi Key |
GNMUEVRJHCWKTO-HXUWFJFHSA-N
|
| InChi Code |
InChI=1S/C25H22ClN5O2S/c1-13-14(2)34-25-22(13)23(16-4-6-17(26)7-5-16)28-20(24-30-29-15(3)31(24)25)12-21(33)27-18-8-10-19(32)11-9-18/h4-11,20,32H,12H2,1-3H3,(H,27,33)/t20-/m1/s1
|
| 化学名 |
2-[(9R)-7-(4-chlorophenyl)-4,5,13-trimethyl-3-thia-1,8,11,12-tetrazatricyclo[8.3.0.02,6]trideca-2(6),4,7,10,12-pentaen-9-yl]-N-(4-hydroxyphenyl)acetamide
|
| 别名 |
(R)-OTX-015; (R)-MK-8628; (R)-Birabresib; 1983196-25-3; 2-((6R)-4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)-N-(4-hydroxyphenyl)acetamide; (R)-2-(4-(4-Chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)-N-(4-hydroxyphenyl)acetamide; 2-[(9R)-7-(4-chlorophenyl)-4,5,13-trimethyl-3-thia-1,8,11,12-tetrazatricyclo[8.3.0.02,6]trideca-2(6),4,7,10,12-pentaen-9-yl]-N-(4-hydroxyphenyl)acetamide; starbld0038411; SCHEMBL23728706;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 200 mg/mL (406.51 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 5 mg/mL (10.16 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 50.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 5 mg/mL (10.16 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 50.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 5 mg/mL (10.16 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0326 mL | 10.1628 mL | 20.3256 mL | |
| 5 mM | 0.4065 mL | 2.0326 mL | 4.0651 mL | |
| 10 mM | 0.2033 mL | 1.0163 mL | 2.0326 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02259114 | Completed Has Results | Drug: Birabresib | NUT Midline Carcinoma Triple Negative Breast Cancer |
Oncoethix GmbH, a subsidiary of Merck & Co., Inc. (Rahway, New Jersey USA) |
October 23, 2014 | Phase 1 |
| NCT02698189 | Terminated Has Results | Drug: Birabresib Dose 20 mg | AML Including AML de Novo and AML Secondary to MDS DLBCL |
Merck Sharp & Dohme LLC | May 19, 2016 | Phase 1 |
| NCT02296476 | Terminated Has Results | Drug: Birabresib | Glioblastoma Multiforme | Oncoethix GmbH, a subsidiary of Merck & Co., Inc. (Rahway, New Jersey USA) |
October 29, 2014 | Phase 2 |
| NCT02698176 | Terminated Has Results | Drug: Birabresib | NUT Midline Carcinoma (NMC) Triple Negative Breast Cancer (TNBC) |
Merck Sharp & Dohme LLC | May 4, 2016 | Phase 1 |