| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
FXa (IC50 = 0.7 nM); FXa (Ki = 0.4 nM)
|
|---|---|
| 体外研究 (In Vitro) |
一种名为利伐沙班 (BAY 59-7939) 的口服直接 Xa 因子 (FXa) 抑制剂正在开发中,用于治疗和预防静脉和动脉血栓形成。 Rivaroxaban 竞争性抑制凝血酶原酶活性 (IC50 2.1 nM) 和人 FXa (Ki 0.4 nM),其选择性比其他丝氨酸蛋白酶高 >10,000 倍。与大鼠血浆(IC50 290 nM)相比,人和兔血浆表现出利伐沙班对内源性 FXa 更有效的抑制作用(IC50 21 nM)。在人血浆中,它表现出抗凝特性,在 0.69 μM 时激活部分凝血活酶时间并增加凝血酶原时间 (PT)[2]。
|
| 体内研究 (In Vivo) |
利伐沙班 (BAY 59-7939) 是一种强效、特异性直接 FXa 抑制剂,具有良好的口服吸收和体内作用[1]。当在血栓诱导前静脉推注时,利伐沙班 (BAY 59-7939) 可减少血栓形成 (ED50 0.1 mg/kg),抑制 FXa,并以剂量依赖性方式延长 PT。在 ED50 时,PT 和 FXa 有微小变化(分别增加 1.8 倍和抑制 32%)。 0.3 mg/kg 的剂量几乎完全阻止血栓形成,利伐沙班表现出 PT 的中度延长(3.2±0.5 倍)和 FXa 活性的抑制(65±3%)[2]。
|
| 酶活实验 |
体外研究:[1]
FXa和相关丝氨酸蛋白酶。在25°C的96孔微量滴定板中使用显色或荧光底物测量对人FVIIa、FIXa、FXa、FXIa、凝血酶、纤溶酶、胰蛋白酶、尿激酶和活化蛋白C的酶活性。将酶与测试化合物或其溶剂(DMSO)孵育10分钟,并通过添加适当的底物引发反应。通过Spectra Rainbow Thermo Reader在405nm处连续监测颜色变化,并通过SPECTRAFluor Plus微孔板读取器在360/465nm处测量荧光。将底物和酶溶解在坐浴盆或适当的测定缓冲液中。 凝血酶原时间(PT)测定。[1] 使用市售试剂盒测量PT。根据制造商的说明,在凝血仪中测量凝结时间。将浓度增加的抑制剂或溶剂添加到血浆中,并在37°C下孵育10分钟。测量凝结时间,并将其与来自适当对照血浆的凝结时间进行比较。 玻璃体蛋白结合。[1] 通过平衡透析法(Scholtan 1962)评估体外血浆蛋白结合。将[14C]-5添加到大鼠、狗和人血浆的每个等分试样中,以使目标浓度为0.1、1.0、3.0、10、30和100 mg L-1;此外,制备了400mg L-1的目标浓度,但仅用于人血浆。在37°C下孵育15分钟后,在装有0.8 mL由纤维素膜(Diachema 10.14纤维素膜,MWCO 5000 kDa)分离的Teflon半细胞的平衡透析器中,用等体积的磷酸盐缓冲等渗溶液(PBS,pH 7.4)在37℃下透析0.8 mL加标血浆1小时。通过LSC测定缓冲液和血浆中[14C]-5的放射性。未结合的5的分数(fu[%])计算如下: fu=cu/c×100,其中cu是未结合的5的浓度,c是5的总浓度。 View More
X射线晶体学。[1] 酶测定[2] BAY 59-7939对纯化丝氨酸蛋白酶的活性是在25 °C。将酶与BAY 59-7939或其溶剂二甲基亚砜(DMSO)孵育10 min。通过加入底物引发反应,并在405处连续监测颜色或荧光 nm,使用Spectra Rainbow Thermo Reader,或630/465 nm,分别使用SPECTRAfluor plus(Tecan)进行20 min(如果未另行说明)。 在以下缓冲液(最终浓度)中分析酶活性:人FXa(0.5 nm)、兔FXa(2 nm)、大鼠FXa(10 nm)或尿激酶(4 nm)在50 mm Tris–HCl缓冲液,pH 8.3, 150 mm NaCl和0.1%牛血清白蛋白(BSA);Pefachrome FXa(50–800 µm)或chromozym U(250 µm)与凝血酶(0.69 nm)、胰蛋白酶(2.2 nm)或纤溶酶(3.2 nm),单位为0.1 µm Tris–HCl,pH 8.0和20 mm CaCl2;色酶TH(200 µm),色酶纤溶酶(500 µm),或色酶胰蛋白酶(500 µm)与FXIa(1 nm)或APC(10 nm)在50 mm磷酸盐缓冲液,pH 7.4, 150 mm NaCl;和S 2366(150或500 µm)与FVIIa(1 nm)和组织因子(3 nm)在50 mm Tris–HCl缓冲液,pH 8.0, 100 mm NaCl,5 mm CaCl2和0.3%BSA、H-D-Phe-Pro-Arg-6-氨基-1-萘-苄基磺酰胺·H2O(100 µm),并测量3 h,如前所述。FIXaβ/FX测定,包括FIXaβ(8.8 nm)和FX(9.5 nm)在50 mm Tris–HCl缓冲液,pH 7.4, 100 mm NaCl,5 通过加入I-1100(50 µm),并测量60 最小。 血浆中FXa活性[2] 人、大鼠或兔血浆(45 µL)与5 µL水蛭素(10 µg mL−1),5 µL BAY 59-7939或二甲基亚砜,和50 µL RVV(人,0.7 mU mL−1;大鼠/兔子,3.5 mU mL−1),溶于50 37µm CaCl2 °C。染色体X(50 µL;600 µm) 分钟。光密度的增加是在37 °C,如上所述。 凝血测定[2] 使用市售试剂盒测量活化部分凝血活酶时间(aPTT)和凝血酶原时间(PT)。BAY 59-7939或DMSO(3 µL)添加到100 µL贫血小板血浆(PPP)并孵育10 最小37 °C。根据制造商的说明(最终卷303),在凝血仪中测量凝结时间 µL)。抗凝活性定义为使血浆凝固时间增加一倍所需的浓度[CT2(µm)]。 血浆制备[2] 通过静脉穿刺从过去10年中未服药的健康受试者身上采集人体血液 天。通过穿刺颈动脉瘤获得兔血,并在麻醉下从腹主动脉抽取大鼠血。将血液收集到含有1/10体积的3.8%柠檬酸三钠的塑料管中。通过在2500下立即离心获得PPP 10的g 最小值为4 °C,并储存在− 20 °C。 |
| 动物实验 |
Dssolved in polyethylene glycol/H2O/ glycerol (996 g/100 g/60 g) (for i.v.); and dissolved in solutol/ethanol/H2O [40%/10%/50% (v/v/v)] (for p.o.);
≤0.3 mg/kg for both i.v. and for p.o.; i.v. injection or Oral gavage; Fasted, male Wistar rats (HsdCpb:WU) and fasted, female New Zealand White rabbits (Esd:NZW). In Vivo Studies: [1] Arteriovenous (AV) Shunt Model. The antithrombotic activity was determined in an AV shunt in anesthetized rats, as described previously 21 with minor modifications: The right common carotid artery and the left jugular vein were cannulated with two 100 mm-long, saline-filled, polyethylene catheters. The catheters were connected with a 30 mm-long polyethylene tube containing a rough nylon thread (40 mm × 0.15 mm), folded to create a 20 mm-long double string. The test compound dissolved in poly(ethylene glycol)/water/glycerol (996 g/100 g/60 g) or vehicle was given by intravenous bolus injection into a tail vein 10 min before thrombus induction. Alternatively, the test compound dissolved in solutol/ethanol/water (40%/10%/50% [v/v/v]) or vehicle was administered orally 90 min before thrombus induction. The shunt was opened for 15 min, and the nylon thread covered with the thrombus was then withdrawn and weighed. Blood samples were withdrawn from the carotid artery just after thrombus removal. View More
Rat venous stasis model [2] Arteriovenous shunt model in rats and rabbits [2] An arteriovenous (AV) shunt in anesthetized rats and rabbits was performed as described previously, with minor modifications. The right common carotid artery and the left jugular vein were cannulated with two 100-mm-long, saline-filled catheters. In rats (n = 10 per dose group), the polyethylene catheters were connected with a 30-mm-long polyethylene tube containing a rough nylon thread (40 × 0.15 mm), folded into a double string. In rabbits (n = 6 per dose group), polyurethane vein catheters (outside diameter 2.1 mm) were connected with a 40-mm-long polyethylene tube, containing a rough nylon thread (60 × 0.15 mm), folded into a double string. BAY 59-7939, dissolved in solutol/ethanol/H2O [40%/10%/50% (v/v/v)], or vehicle was given orally 90 min before the shunt was opened for 15 min. The nylon thread was then withdrawn and weighed. Blood samples were withdrawn from the carotid artery just after thrombus removal. Rat tail-bleeding model[2] BAY 59-7939 (n = 10 per dose group) or vehicle was given orally 90 min before the tails of anesthetized rats were transected 2 mm from the tip and vertically immersed in saline at 37 °C. The time until continuous blood flow ceased for > 30 s was measured, with a maximum observation time of 10 min (longer bleeding times were assigned a value of 10 min). Rabbit ear-bleeding model[2] Ear-bleeding time (EBT) was determined in anesthetized rabbits (n = 5 per dose group), as described previously. A standardized 3-mm-long incision was made at different sites of the right ear in each animal 90 and 105 min after administration of oral BAY 59-7939 or vehicle. Blood from the incision was removed with filter paper every 30 s. The time until the bleeding stopped was measured. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following oral administration, rivaroxaban is rapidly absorbed and reaches peak plasma concentration in 2-4 hours. Bioavailability of the 10 mg dose is >80%. However, the 15-20 mg dose have a lower bioavailability if taken in the fasted state and consequently should be taken with food. Approximately two-thirds of rivaroxaban is excreted into urine (via active tubular secretion in which approximately 36% as unchanged drug and 30% as inactive metabolism). The remaining third of the administered dose is excreted via feces in which 7% is in the form of unchanged drug and 21% as inactive metabolites. The steady state Vd is 50 L Systemic clearance is approximately 10 L/h, so rivaroxaban is considered a drug with low clearance. Renal clearance is ~3-4 L/h. Following oral administration, approximately one-third of the absorbed dose is excreted unchanged in the urine, with the remaining two-thirds excreted as inactive metabolites in both the urine and feces. In a Phase 1 study, following the administration of a (14)C-rivaroxaban dose, 66% of the radioactive dose was recovered in urine (36% as unchanged drug) and 28% was recovered in feces (7% as unchanged drug). Unchanged drug is excreted into urine, mainly via active tubular secretion and to a lesser extent via glomerular filtration (approximate 5:1 ratio). Rivaroxaban is a substrate of the efflux transporter proteins P-gp and ABCG2 (also abbreviated Bcrp). Rivaroxaban's affinity for influx transporter proteins is unknown. Plasma protein binding of rivaroxaban in human plasma is approximately 92% to 95%, with albumin being the main binding component. The steady-state volume of distribution in healthy subjects is approximately 50 L. Absorption of rivaroxaban is dependent on the site of drug release in the GI tract. A 29% and 56% decrease in AUC and Cmax compared to tablet was reported when rivaroxaban granulate is released in the proximal small intestine. Exposure is further reduced when drug is released in the distal small intestine, or ascending colon. Avoid administration of rivaroxaban distal to the stomach which can result in reduced absorption and related drug exposure. The absolute bioavailability of rivaroxaban is dose-dependent. For the 10 mg dose, it is estimated to be 80% to 100% and is not affected by food. Xarelto 10 mg tablets can be taken with or without food. For the 20 mg dose in the fasted state, the absolute bioavailability is approximately 66%. Coadministration of Xarelto with food increases the bioavailability of the 20 mg dose (mean AUC and Cmax increasing by 39% and 76% respectively with food). Xarelto 15 mg and 20 mg tablets should be taken with food. For more Absorption, Distribution and Excretion (Complete) data for Rivaroxaban (8 total), please visit the HSDB record page. Metabolism / Metabolites Approximately two-thirds of the dose is metabolized. It is metabolized by CYP3A4, CYP3A5, CYP2J2 and CYP-independant mechanisms Rivaroxaban undergoes oxidative degradation by cytochrome P-450 (CYP) isoenzymes 3A4/5 and 2J2 and hydrolysis; metabolites are subsequently eliminated through renal and fecal/biliary routes. No major circulating metabolites have been identified in plasma. Biological Half-Life The terminal half life is 5-9 hours in adults and 11-13 hours in the elderly. The terminal elimination half-life is 11 to 13 hours in the elderly. The terminal elimination half-life of rivaroxaban is 5 to 9 hours in healthy subjects aged 20 to 45 years. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Chronic therapy with rivaroxaban is associated with moderate ALT elevations (greater than 3 times the upper limit of normal) in 1.5% to 3% of patients, an overall rate which is slightly lower than with low molecular weight heparins and similar to the rates with warfarin. During the large, prelicensure clinical trials of rivaroxaban, several instances of ALT elevations with jaundice occurred, but few details were provided and it was not clear whether the liver injury was clinically apparent. The cases were evidently mild and self-limited, resolving completely once therapy was stopped. Since its licensure and more wide scale use, rivaroxaban has been linked to many instances of acute liver injury with jaundice. The clinical features of these cases varied widely. Most cases had an onset within 1 to 8 weeks of starting rivaroxaban and presented with jaundice, fatigue and a hepatocellular pattern of serum enzyme elevations. In some individuals, a cholestatic or mixed pattern was found. Immunoallergic features and autoimmune markers were atypical but at least one case occurred with skin rash and fever suggestive of DRESS syndrome. One case of acute hepatic necrosis and death attributed to rivaroxaban has been reported, but ischemic hepatitis due to severe heart failure was a more likely cause of the acute liver failure. All other reported cases of rivaroxaban induced liver injury recovered upon stopping rivaroxaban, usually quite promptly, within 2 to 4 weeks. In large health care databases, hospitalization for acute liver injury arises in approximately 1 in 2,200 cases, but whether all cases in these databases represent liver injury from rivaroxaban is uncertain. Likelihood score: A (well established cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Several case reports and one thorough pharmacokinetic analysis consistently indicate that maternal doses of rivaroxaban of 15 to 30 mg daily produce low levels in milk that are considerably below doses (<2%) required for anticoagulation in infants. Plasma rivaroxaban levels in two breastfed infants were undetectable. If the mother requires rivaroxaban, it is not a reason to discontinue breastfeeding. ◉ Effects in Breastfed Infants A 38-year-old woman with antiphospholipid syndrome began rivaroxaban 15 mg (0.19 mg/kg) daily at 5 days postpartum for prophylaxis of deep vein thrombosis. She partially breast-fed her infant (at least 50%). No apparent evidence of bleeding was noted in the infant at 1- and 3-month check-ups and development was normal at 18 months of age. Two mothers received rivaroxaban 15 mg (0.22 and 0.25 mg/kg) daily beginning 3 days postpartum for prophylaxis of deep vein thrombosis. At 3 months postpartum, their infants continued to be breastfed (extent not stated) and had no health problems or bleeding events. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Plasma protein binding is about 92% to 95% Interactions Patients with renal impairment receiving full dose Xarelto in combination with drugs classified as combined P-gp and weak or moderate CYP3A4 inhibitors (e.g., amiodarone, diltiazem, verapamil, quinidine, ranolazine, dronedarone, felodipine, erythromycin, and azithromycin) may have increases in exposure compared with patients with normal renal function and no inhibitor use, since both pathways of rivaroxaban elimination are affected. Xarelto should be used in patients with CrCl 15 to 50 mL/min who are receiving concomitant combined P-gp and weak or moderate CYP3A4 inhibitors only if the potential benefit justifies the potential risk. Single doses of enoxaparin and Xarelto given concomitantly resulted in an additive effect on anti-factor Xa activity. Single doses of warfarin and Xarelto resulted in an additive effect on factor Xa inhibition and PT. Concomitant aspirin use has been identified as an independent risk factor for major bleeding in efficacy trials. NSAIDs are known to increase bleeding, and bleeding risk may be increased when NSAIDs are used concomitantly with Xarelto. Coadministration of the platelet aggregation inhibitor clopidogrel and Xarelto resulted in an increase in bleeding time for some subjects. Avoid concurrent use of Xarelto with other anticoagulants due to increased bleeding risk unless benefit outweighs risk. Promptly evaluate any signs or symptoms of blood loss if patients are treated concomitantly with aspirin, other platelet aggregation inhibitors, or NSAIDs. Results from drug interaction studies and population PK analyses from clinical studies indicate coadministration of Xarelto with a combined P-gp and strong CYP3A4 inducer (e.g., rifampicin, phenytoin) decreased rivaroxaban exposure by up to 50%. Similar decreases in pharmacodynamic effects were also observed. These decreases in exposure to rivaroxaban may decrease efficacy. Avoid concomitant use of Xarelto with drugs that are combined P-gp and strong CYP3A4 inducers (e.g., carbamazepine, phenytoin, rifampin, St. John's wort). When data suggest a change in exposure is unlikely to affect bleeding risk (e.g., clarithromycin, erythromycin), no precautions are necessary during coadministration with drugs that are combined P-gp and CYP3A4 inhibitors. Avoid concomitant administration of Xarelto with combined P-gp and strong CYP3A4 inhibitors. For more Interactions (Complete) data for Rivaroxaban (10 total), please visit the HSDB record page. |
| 参考文献 |
[1]. Roehrig S, et al. Discovery of the novel antithrombotic agent 5-chloro-N-({(5S)-2-oxo-3- [4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene- 2-carboxamide (BAY 59-7939): an oral, direct factor Xa inhibitor. J Med Chem. 2005 Sep 22;48(19)
[2]. Perzborn E, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939--an oral, direct Factor Xa inhibitor. J Thromb Haemost. 2005 Mar;3(3):514-21. |
| 其他信息 |
Therapeutic Uses
Anticoagulant Xarelto is indicated for the treatment of deep vein thrombosis (DVT). /Included in US product label/ Xarelto is indicated to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. There are limited data on the relative effectiveness of Xarelto and warfarin in reducing the risk of stroke and systemic embolism when warfarin therapy is well-controlled. /Included in US product label/ Xarelto is indicated for the prophylaxis of deep vein thrombosis, which may lead to pulmonary embolism in patients undergoing knee or hip replacement surgery. /Included in US product label/ For more Therapeutic Uses (Complete) data for Rivaroxaban (7 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ WARNING: PREMATURE DISCONTINUATION OF XARELTO INCREASES THE RISK OF THROMBOTIC EVENTS. Premature discontinuation of any oral anticoagulant, including Xarelto, increases the risk of thrombotic events. If anticoagulation with Xarelto is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant /BOXED WARNING/ WARNING: SPINAL/EPIDURAL HEMATOMA. Epidural or spinal hematomas have occurred in patients treated with Xarelto who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include: use of indwelling epidural catheters; concomitant use of other drugs that affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants; a history of traumatic or repeated epidural or spinal punctures a history of spinal deformity or spinal surgery. Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary. Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis Rivaroxaban increases the risk of hemorrhage and can cause serious or fatal bleeding. Bleeding complications were the most common adverse effects of rivaroxaban reported in clinical trials. Use of rivaroxaban should be avoided in patients with moderate (Child-Pugh class B) or severe (Child-Pugh class C) hepatic impairment or with any hepatic disease associated with coagulopathy; systemic exposure and risk of bleeding may be increased in such patients. For more Drug Warnings (Complete) data for Rivaroxaban (13 total), please visit the HSDB record page. Pharmacodynamics Rivaroxaban is an anticoagulant which binds directly to factor Xa. Thereafter, it effectively blocks the amplification of the coagulation cascade, preventing the formation of thrombus. Rivaroxaban is a unqiue anticoagulant for two reasons. First of all, it is does not involve antithrombin III (ATIII) to exert its anticoagulant effects. Secondly, it is an oral agent whereas the widely used unfractionated heparin and low molecular weight heparins are for parenteral use only. Although the activated partial thromboplastin time (aPTT) and HepTest (a test developed to assay low molecular weight heparins) are prolonged in a dose-dependant manner, neither test is recommended for the assessment of the pharmacodynamic effects of rivaroxaban. Anti-Xa activity and inhibition of anti-Xa activity monitoring is also not recommended despite being influenced by rivaroxaban. |
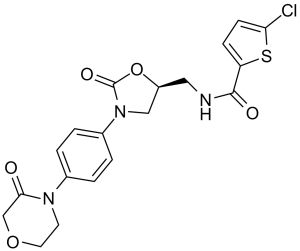
| 分子式 |
C19H18CLN3O5S
|
|---|---|
| 分子量 |
435.88
|
| 精确质量 |
435.065
|
| 元素分析 |
C, 52.35; H, 4.16; Cl, 8.13; N, 9.64; O, 18.35; S, 7.36
|
| CAS号 |
366789-02-8
|
| 相关CAS号 |
Rivaroxaban-d4;1132681-38-9
|
| PubChem CID |
9875401
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
732.6±60.0 °C at 760 mmHg
|
| 熔点 |
228-229°C
|
| 闪点 |
396.9±32.9 °C
|
| 蒸汽压 |
0.0±2.4 mmHg at 25°C
|
| 折射率 |
1.633
|
| LogP |
1.84
|
| tPSA |
116.42
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
29
|
| 分子复杂度/Complexity |
645
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C1COCC(=O)N1C2=CC=C(C=C2)N3C[C@@H](OC3=O)CNC(=O)C4=CC=C(S4)Cl
|
| InChi Key |
KGFYHTZWPPHNLQ-AWEZNQCLSA-N
|
| InChi Code |
InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1
|
| 化学名 |
(S)-5-chloro-N-((2-oxo-3-(4-(3-oxomorpholino)phenyl)oxazolidin-5-yl)methyl)thiophene-2-carboxamide
|
| 别名 |
BAY 59-7939; Rivaroxaban; BAY59-7939; BAY-59-7939; trade name: Xarelto.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (5.74 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.74 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 0.5% methylcellulose+0.2% Tween 80:5 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2942 mL | 11.4710 mL | 22.9421 mL | |
| 5 mM | 0.4588 mL | 2.2942 mL | 4.5884 mL | |
| 10 mM | 0.2294 mL | 1.1471 mL | 2.2942 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06314763 | Recruiting | Drug: Rivaroxaban 20mg Drug: Sotorasib 960mg |
Drug Drug Interaction Study | Radboud University Medical Center | November 9, 2023 | Phase 4 |
| NCT02970773 | Withdrawn | Drug: Rivaroxaban Oral Tablet | Spinal Cord Injuries Thromboembolism |
Swiss Paraplegic Research, Nottwil | December 4, 2017 | Phase 4 |
| NCT05410275 | Not yet recruiting | Drug: Rivaroxaban | Chronic Hemodialysis Patients | University Hospital, Tours | December 1, 2022 | Phase 3 |
| NCT02047006 | Completed | Drug: Rivaroxaban 10 mg | Chronic Renal Failure | AZ Sint-Jan AV | September 2013 | Phase 4 |
|
|---|
|
|