| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 靶点 |
Factor Xa (Ki = 0.08 nM and 0.17 nM for human and rabbit Factor Xa)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
在浓度 (EC2x) 分别为 3.6 μM、0.37 μM、7.4 μM 和 0.4 μM 时,阿哌沙班 (BMS-562247-01) 延长正常人血浆的凝血时间,这是使凝血酶原时间 (PT) 加倍所需的,改良凝血酶原时间 (mPT)、活化部分凝血活酶时间 (APTT) 和 HepTest。此外,在 PT 和 APTT 测试中,阿哌沙班在人和兔血浆中表现出最高的效力,但在大鼠和狗血浆中表现出较低的效力[2]。
选择性和责任分析。[1] 化合物40/阿哌沙班与其他蛋白酶相比显示出高度的选择性(见补充部分),甚至与化合物47a和5.15a相比也是如此。此外,该化合物对各种P450同工酶的活性较弱(IC50>25μM),对hERG钾通道的活性较弱。26a-e化合物40的溶解度约为40-50μg/mL。27在人肝微粒体试验中,化合物40非常稳定,T1/2>100分钟(未测量47的HLM T1/2)。化合物40(Papp=0.9×10-6cm-s-1)和47(Papp=2.5×10-6cm s-1)的Caco-2渗透率值为中等至高。 在体外,阿哌沙班具有强效和选择性,对人FXa的K(i)为0.08 nm。它在FXa抑制[FXa K(i)(nm):0.16,兔;1.3,大鼠;1.7,狗]和抗凝[EC(2x)(微米,凝血酶原时间加倍所需的浓度):3.6,人;2.3,兔;7.9,大鼠,6.7,狗]方面表现出物种差异。10微米的阿哌沙班不会改变人和兔血小板对ADP、γ-凝血酶和胶原蛋白的聚集。[2] 酶测定[2] 阿哌沙班抑制人类FXa的Lineweaver–Burk图表明,阿哌沙班组是一种与显色肽底物S‐2765竞争的抑制剂,Ki为0.08 nm(图1)。阿哌沙班还抑制了大鼠、狗和兔子的FXa(表1)。就25°C下的FXa-Ki而言,阿哌沙班在抑制人和兔FXa方面具有相似的效力,但对大鼠和狗FXa的效力要低10-20倍(表1)。 凝血试验[2] 正如预期的那样,在正常人血浆中添加阿哌沙班可以延长凝血时间,包括APTT、PT、mPT和HepTest。在三种凝血时间测定中,mPT和HepTest在监测阿哌沙班在人血浆中的体外抗凝作用方面的灵敏度似乎是APTT和PT的10-20倍(表1)。在PT和APTT检测中,阿哌沙班在人和兔血浆中的效力最高,但在大鼠和狗血浆中效力较低(表1)。 血小板聚集[2] ADP、γ-凝血酶和胶原蛋白的体外血小板聚集反应平均为47±5%、53±4%和51±5%,在人类PRP中分别为50±5%、56±5%和60±1%,分别在兔PRP中。阿哌沙班在1、3和10μm时没有显著改变这些血小板反应,但在3μm时几乎完全被GPIIb/IIIa拮抗剂DMP802抑制(数据未显示)。 阿哌沙班在兔和人血浆中的体外药效作用[3] 随着血浆阿哌沙班浓度的增加,体外FXa活性降低(图1);兔子和人类的浓度-反应曲线相似。表1总结了从掺有不同浓度阿哌沙班的体外血浆样品中测定的IC50值。兔和人血浆的IC50值相等:0.25μM(±0.01)。之前曾报道过人和兔血浆中PT和mPT加倍所需的血浆浓度。 |
||
| 体内研究 (In Vivo) |
在犬中,阿哌沙班 (BMS-562247-01) 具有良好的药代动力学,具有极低的分布容积(Vdss:0.2 L/kg)和极低的清除率(Cl:0.02 L/kg/h)。此外,阿哌沙班具有良好的口服生物利用度(F:58%)和合理的半衰期(T1/2:5.8 小时)[1]。阿哌沙班在动静脉分流血栓形成 (AVST)、静脉血栓形成 (VT) 和电子介导的颈动脉血栓形成 (ECAT) 兔模型中表现出剂量依赖性抗血栓作用,EC50 值分别为 270 nM、110 nM 和 70 nM[2 ]。在离体兔子中,阿哌沙班显着降低 Xa 因子活性,IC50 为 0.22 μM[3]。阿哌沙班在黑猩猩中还表现出较低的全身清除率(Cl:0.018 L/kg/h)、适度的分布容积(Vdss:0.17 L/kg)和良好的口服生物利用度(F:59%)[4]。
犬药代动力学和兔抗血栓疗效。[1] 由于化合物40/阿哌沙班和47具有优异的体外效力和选择性,因此使用卡式给药范式在狗身上研究了这两种化合物的药代动力学特征(“N-in-one”研究,表6)。7a,34a,b乙酰化N-甲基类似物47具有口服生物可利用性;但显示出高清除率(Cl=2.8 L kg-1 h-1)、中等分布体积(Vdss=1.7 L kg-1)和不可接受的半衰期。化合物40的犬药代动力学表现出色,清除率极低(Cl=0.02 L kg-1 h-1),分布体积小(Vdss=0.2 L kg-1)。这些值明显低于化合物4和5的观察值。FXa是血管靶点,化合物40的药代动力学特征被认为是非常理想的,不太可能产生非靶点相关的不良反应。重要的是,化合物40的半衰期适中(T1/2=5.8小时),口服生物利用度良好(F=58%)。通过平衡透析测量的40人血清蛋白结合率为87%。25在兔AVShont血栓形成模型中(图4),化合物40以剂量依赖的方式抑制血栓形成,IC50值为329 nM。15d,e这与同一模型中化合物4的抗血栓效力(IC50=340 nM)相当。 AVST模型[2] 不同载体处理的AVST兔的平均血栓重量相似,范围为290±11至327±15mg(每组n=6)。如图2所示,阿哌沙班、磺达肝癸钠和华法林对AVST兔有效,并产生剂量依赖性抗血栓作用;表2中报告了它们的ED50值。在该模型中研究的最高剂量下,与相应的赋形剂组相比,阿哌沙班静脉注射3 mg kg−1 h−1、磺达肝癸钠静脉注射1 mg kg−1 h−1和华法林口服3 mg kg-1 d−1分别将血栓重量降低了98%、86%和77%。我们观察到阿哌沙班静脉注射0.03、0.1、0.3、1和3 mg kg−1 h−1时,血浆水平分别线性剂量比例增加34±2、121±9、490±104、1155±153和3705±525 nm(每组n=3-7)。阿哌沙班的EC50估计为357±90 nm。 VT模型[2] 不同赋形剂治疗的室性心动过速兔的平均血栓重量相似,范围为64±2至79±7 mg(每组n=6)。在该模型中,阿哌沙班、磺达肝癸钠和华法林产生了剂量依赖性的抗血栓作用(图2);表2给出了它们的ED50值。在该模型研究的最高剂量下,与相应的赋形剂组相比,阿哌沙班1 mg kg−1 h−1、磺达肝癸钠0.3 mg kg−2 h−l和华法林3 mg kg−1 d−1分别将血栓形成减少了83%、74%和84%。 动脉血栓形成模型图3(上图)显示了电刺激后赋形剂和阿哌沙班对颈动脉血流的影响。接受赋形剂治疗的动物的基础颈动脉血流量平均为21±4 mL min−1。在血栓形成开始后,血流逐渐减少,在约35分钟内,接受赋形剂治疗的动物动脉完全闭塞。静脉注射阿哌沙班0.01-1 mg kg−1 h−1时,损伤动脉的通畅时间呈剂量依赖性增加。静脉注射0.03–1 mg kg−1 h−1时,任何动物在90分钟内都没有闭塞。 体外凝血标志物[2] 图4显示了从AVST、VT和ECAT研究中获得的阿哌沙班、磺达肝癸钠和华法林的体外APTT和PT反应总结。阿哌沙班在3 mg kg−1 h−1的剂量下显著延长了离体APTT,在0.3 mg kg−1 h−1及以上的剂量下显着延长了PT(图4)。在所研究的剂量下,磺达肝癸钠对离体APTT和PT没有显著影响。华法林在0.3 mg kg−1天及以上的剂量下显著延长了离体APT,在0.1 mg kg−1天及以下的剂量下延长了PT(图4)。 表皮出血时间模型[2] 阿哌沙班、磺达肝癸钠和华法林赋形剂治疗组的平均角质层BT分别为172±2、181±7和183±7秒(每组n=6)。口服0.1、0.3、1和3 mg kg−1天-1的华法林会导致BT的剂量依赖性增加(分别为228±14、371±24、929±70和1129±43秒),ED3×为0.70 mg kg−1天-1(表2)。与赋形剂相比,口服1和3 mg kg-1天-1的华法林分别使BT显著增加了5.1倍和6.2倍(p < 0.05). 相比之下,抗血栓剂量的磺达肝癸钠和阿哌沙班的BT略有增加(静脉注射0.3、1和3 mg kg−1 h−1的磺达肾癸钠分别为166±4、210±12和213±11 s;阿哌沙班,静脉注射1和3 mg kg−1 h−1时分别为191±8和228±14秒)。静脉注射3 mg kg−1 h−1时,磺达肝癸钠和阿哌沙班的BT分别比其赋形剂增加了1.3倍和1.2倍,两种化合物的ED3×均大于3 mg kg-1 h−l(表2)。 阿哌沙班对人和兔因子Xa(FXa)具有相似的亲和力。兔子通常用于血栓形成疾病模型的开发;然而,与其他物种不同,阿哌沙班在兔子体内表现出较差的口服生物利用度(F=3%)和较高的清除率(2.55 l/h/kg)。兔肝微粒体对[14C]阿哌沙班的氧化代谢速度约为大鼠或人类的20倍。静脉注射(IV)剂量为5mg/kg后,[14C]阿哌沙班的循环水平从最早的采样时间(5分钟)降至4小时无法检测到。口服剂量为30mg/kg后,仅在1和4小时检测到[14C]阿哌沙班水平。放射性分析显示,阿哌沙班仅在静脉注射后才成为血浆中的重要成分;静脉注射和口服后,O-去甲基阿哌沙班(M2)、O-去甲基阿哌沙班葡萄糖醛酸(M14)和O-去甲基硫酸阿哌沙班酯(M1)是主要的代谢产物。阿哌沙班在兔体内的研究表明,阿哌沙班浓度与FXa活性的抑制、凝血酶原时间的延长和凝血酶原时间改变之间存在良好的相关性,这些体外药效学标志物与血浆药物水平之间没有滞后时间。阿哌沙班离体抑制FXa活性50%(IC50)所需的浓度(0.22+/-0.02微M)与兔和人血浆体外实验的IC50一致。总之,阿哌沙班对人类和兔子的FXa显示出相似的亲和力。它产生快速起效、可预测的浓度依赖性药效反应,并且与大鼠或人类不同,它在兔子体内具有快速的肝脏代谢。[3] 阿哌沙班是一种强效、高选择性、可逆的口服直接Xa因子(fXa)抑制剂,正在开发用于血栓预防和治疗。阿哌沙班的临床前药代动力学(PK)属性具有分布体积小(Vd)、全身清除率低(CL)和良好的口服生物利用度阿哌沙班在大鼠、狗和黑猩猩体内吸收良好,绝对口服生物利用度约为50%或更高。阿哌沙班在大鼠、狗和黑猩猩体内的稳态Vd约为0.5、0.2和0.17 l/kg,而CL分别约为0.9、0.04和0.018 l/h/kg。阿哌沙班的体外代谢清除率也很低。肾清除率约占大鼠、狗和黑猩猩全身清除率的10-30%。大鼠、狗和黑猩猩的抗fXa活性、凝血酶原时间(PT)和HEPTEST(®)凝血时间(HCT)延长与血浆阿哌沙班浓度密切相关。阿哌沙班血药浓度与药效学(PD)标志物之间没有滞后时间,表明阿哌沙班起效迅速。PK/PD分析使用抑制性E(max)模型进行抗fXa测定,使用线性模型进行PT和HCT测定。大鼠和狗的抗fXa活性IC(50)值分别为0.73±0.03和1.5±0.15μM。大鼠、狗和黑猩猩的PT表观K(i)值分别约为1.7、6.6和4.8μM。狗的HCT表观K(i)约为1.3μM。阿哌沙班在临床开发中表现出理想的PK和PD特性,具有良好的口服生物利用度、小Vd、低CL和直接、可预测、浓度依赖的PD反应[4]。 |
||
| 酶活实验 |
酶亲和力测定。[1]
所有酶Ki值均来自纯化的人酶。所有fXa测定均在微量滴定板中进行,使用总体积为250μL的0.1 M磷酸钠缓冲液,该缓冲液含有0.2 M NaCl和0.5%聚乙二醇6000,pH值为7.0。化合物在10、3.16、1.0、0.316、0.1、0.0316、0.01和0.003 16μM下运行。在405nm下读取板30分钟。在对照组(无抑制剂)和抑制剂存在的情况下测定速率。根据这些速率确定酶活性百分比,并在以下公式中用于确定Ki: 其中S是底物浓度,ACT是抑制剂速率的酶活性百分比分数。所有化合物都在重复研究中进行了测试,并与相同的内标进行了比较。批内和批间变异性分别为5%和20%。参考文献28和29详细描述了这些测定。所有酶测定均在室温下在pH 7.4缓冲液中进行。所有酶均从人体组织中纯化,并从市售来源获得。在单独的实验中测定了单个酶和底物Km,接近文献中确定的值。通过将一系列抑制剂浓度(1 nM至50μM,一式两份)与固定酶(0.1−100 nM)和肽底物(200−1000μM)浓度一起孵育30分钟来确定酶活性的稳态抑制。根据IC50或每种抑制剂浓度的抑制程度,假设竞争性抑制和一个位点结合,计算Ki。 体外凝血试验(PT/APT)。[1] 标准凝血试验在温度控制的自动凝血装置中进行。通过静脉穿刺从健康志愿者身上采集血液,并用1/10体积的0.11M缓冲柠檬酸钠抗凝。在2000g下离心10分钟后获得血浆,并在使用前置于冰上。在DMSO中制备10mM的抑制剂初始储备溶液。随后在血浆中进行稀释。在分析之前,将含有抑制剂的血浆溶液置于冰上。在对照血浆和含有5至7种不同浓度抑制剂的血浆上测定凝血时间。每种血浆浓度的测定一式两份。将每种浓度的凝血时间与每种合并血浆的对照凝血时间进行比较。根据试剂说明,使用Dade凝血活酶C Plus进行凝血酶原时间测试。在加入Dade凝血活酶C Plus(100μL)之前,将血浆(50μL)加热至37°C 3分钟。根据试剂说明,使用AlexinTM进行活化部分凝血活酶时间(aPTT)。在加入aPTT试剂(50μL)之前,将血浆(50μL)加热至37°C 1分钟。三分钟后加入氯化钙(50μL)。 FXa活性的测定[3] 使用市售的含有测定缓冲液、罗素蝰蛇毒液、氯化钙和FXa显色底物的因子X试剂盒测量FXa活性。所有试剂均按照包装说明书进行制备和使用。缓冲液为50 mM Tris缓冲液(pH 7.8),含有20 mg/l聚异戊二烯(溴化六二甲基林)。对于离体样本,将每个时间点的兔血浆稀释21倍(10μl+200μl缓冲液)。对于体外样品,将合并的正常人或兔血浆加入,使阿哌沙班的最终浓度达到0.1至12.8μM,然后用测定缓冲液稀释21倍。制备不含抑制剂的额外血浆稀释液,以确认FXa活性与添加的血浆量成正比。在96细胞微量滴定板(Costar 3474;Corning股份有限公司,Lowell,MA)中,加入50μl稀释血浆的等分试样,并在读数器内于37°C下孵育10分钟。然后加入50μl因子X底物的等分试样,并在37°C下在平板读数器内孵育4分钟。通过加入50μl罗素蝰蛇毒液/钙引发反应,该毒液/钙在水浴中预热至37°C。底物的水解导致对硝基苯胺的释放,通过分光光度法监测,每15秒测量一次405nm处的吸光度增加,持续20分钟。吸光度变化率表示为mOD/min(每分钟光密度变化1000倍),与酶活性成正比。底物水解的初始速率用于分析。 抗凝血活性测定[3] 根据制造商的指示,使用自动凝血分析仪进行凝血酶原时间(PT)测定。传统的PT测定法测量通过外源途径引发的整体凝血级联反应。对于该测定,将50μl血浆的等分试样在37°C下孵育2分钟,然后加入100μl PT试剂。通过用1.25ml 100mM氯化钙稀释1ml凝血活酶C Plus并使用该稀释试剂代替正常PT试剂来进行改良PT(mPT)测定。凝血时间由自动分析仪记录。大于120秒的凝血时间设定为120秒。 蛋白质结合的测定[3] 平衡透析用于测定兔和人血清中阿哌沙班的蛋白质结合。所有血清在室温下解冻,在2000×g下离心10分钟,以去除残留的凝结蛋白。透析膜用水预处理,随后用0.133M磷酸钾缓冲液(pH 7.4)预处理。将含有1、3或10μM阿哌沙班(0.75ml)的血清加入细胞的一侧,并将等体积的缓冲液(0.133M磷酸钾缓冲液,pH 7.4)加入另一侧。通过在37°C下以3-5rpm的速度旋转细胞3小时来实现平衡。孵育后,分别收集血清和缓冲液侧的等分试样,并与等体积的相反基质混合。 酶测定[2] 使用既定的蛋白质纯化程序,从健康狗、大鼠和兔子的柠檬酸血浆中分离出FX。用罗素蝰蛇毒激活后,通过亲和层析获得纯化的FXa。通过十二烷基硫酸钠聚丙烯酰胺凝胶电泳判断,所得FXa的纯度>95%。使用显色底物S-2765测定人、兔、大鼠和狗FXa的底物亲和力值,表示为米氏常数(Km),分别为36、60、240和70μm。通过使用SpectraMax 384 Plus平板读数器和SoftMax在25°C下测量405nm处的吸光度长达30分钟来监测底物水解。两次测定每种底物和抑制剂浓度对的FXa活性。Ki值是通过使用GRAFIT将稳态底物水解速率非线性最小二乘拟合到竞争性抑制方程(方程1)来计算的,其中v等于反应速度(OD min−1),Vmax等于最大反应速度,S等于底物浓度,I等于抑制剂浓度。 |
||
| 细胞实验 |
凝血试验[2]
在含有1/10体积3.2%柠檬酸钠的试管中采集血液样本,在>2000×g离心10分钟后获得贫血小板血浆。用自动凝血分析仪测量凝血时间。PT、APTT和HepTest试剂已重新配制,并按照制造商的说明进行了检测。通过用1.25 mL 100 mm氯化钙稀释1 mL凝血活酶C Plus,并使用该稀释试剂代替正常PT试剂,进行改良PT(mPT)测定。对于PT和mPT,在加入PT试剂(100μL)之前,将血浆(50μL)加热至37°C 3分钟。对于APTT,在加入APTT试剂(50μL)之前,将血浆(50μL)加热至37°C 1分钟。再过两分钟,加入25mm氯化钙(50μL)。对于HepTest,在加入牛FXa(50μL)之前,将血浆(50μL)加热至37°C 2分钟。再过两分钟,加入HepTest ReCal混合物(50μL)。测定一式两份,表示为治疗组与基线对照组的平均比值。将凝血时间延长2倍所需的浓度(EC2×)表示为总血浆浓度,而不是添加凝血测定试剂后的最终测定浓度。对于体外研究,从10mm二甲亚砜储备溶液开始,将阿哌沙班连续稀释到从健康狗、大鼠和兔子身上获得的柠檬酸血浆中。 血小板聚集试验[2] 用血小板聚集仪在体外测量柠檬酸人和兔富血小板血浆(PRP)中的血小板聚集。在250×g离心6分钟后,从柠檬酸盐血液中获得PRP。将柠檬酸盐PRP(250μL)与20μL赋形剂、3μm DMP802或1-10μm阿哌沙班混合,在37°C下孵育3分钟。DMP802是一种糖蛋白(GP)IIb/IIIa受体拮抗剂,被列为阳性对照(对10μm ADP的人血小板聚集反应的IC50=29 nm)。在加入20μL激动剂(ADP为10μm,γ-凝血酶为35nm,胶原蛋白为10μg mL-1,终浓度)后测定血小板聚集峰值。 阿哌沙班浓度的测定和放射性测量[3] 使用液相色谱-串联质谱(LC-MS/MS)测定了非放射性标记阿哌沙班研究中血浆和其他生物基质中阿哌沙班组的浓度。血浆、尿液和粪便样本中的放射性水平通过液体闪烁计数(LSC)测定。A0387型样品氧化器用于燃烧样品。为了量化放射性,用Carbo-Sorb E捕获产生的14CO2,并将其与Permafluor E+闪烁液混合。 代谢物谱、定量和鉴定[3] 在岛津LC-10AT系统上进行HPLC样品分析,该系统配备有光电二极管阵列紫外检测器和Ace 3,C18(3μm),150×4.6 mm柱。流动相流速为0.7 ml/min。使用岛津二极管阵列检测器通过紫外光谱确认了阿哌沙班和M2的保留时间。为了定量放射性,使用Gilson Model 204馏分收集器以0.26-min的间隔收集HPLC流出物。将板在Automatic Environmental Speed Vac中干燥,并使用Packard TopCount NXT微孔板闪烁和发光计数器对放射性进行计数10分钟。使用Microsoft®Excel软件从TopCount数据重建放射性色谱图 使用Finnigan LTQ离子阱质谱仪和安捷伦HPLC通过LC-MS/MS分析尿液和合并血浆和粪便样本的提取物。使用ESI探针在正离子模式下进行LC-MS分析。将HPLC洗脱液分开,使25%的洗脱液被引导至质谱仪。将流量从0转移到5分钟。将洗脱液流从5分钟引导到质谱仪,直到HPLC运行结束。用于分析的毛细管温度设定为230°C。调整氮气流量、喷雾电流和电压,以获得阿哌沙班的最大灵敏度。 体外培养[3] 将总共10μM[14C]阿哌沙班(静脉注射后的最大浓度[Cmax])与兔、大鼠和人类受试者的混合肝微粒体(1 mg/ml蛋白质浓度,BD Biosciences,Woburn,MA)在含有1 mM烟酰胺腺嘌呤二核苷酸磷酸盐的100 mM磷酸盐缓冲液(pH 7.4)中在37°C下孵育60分钟。用乙腈沉淀蛋白质后,通过HPLC、馏分收集、放射性测量和LC-MS分析对样品进行分析。 |
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Apixaban is approximately 50% bioavailable though other studies report 43-46% oral bioavailability. 56% of an orally administered dose is recovered in the feces and 24.5-28.8% of the dose is recovered in the urine. 83-88% of the dose recovered in the urine was the unchanged parent compound. Approximately 21L. 3.3L/h though other studies report 4876mL/h. Distribution in pregnant rats/fetuses: Cmax in amnion was high. Significant concentrations were found in placenta and fetal blood, kidney and liver. Toxicokinetic data collected in the reproductive and developmental toxicity studies in rats, mice and rabbits showed that generally fetal plasma concentrations of apixaban were lower than those in the dams. Two single dose radiolabel distribution studies were provided in rats. The data show a wide distribution, with the highest values in excretory organs (liver, kidney, urinary bladder (and contents), bile) and intestinal tract (and contents). After a dose of 20 mg/kg in male Long-Evans rats also relatively high Cmax and AUC were found in adrenals, lungs, thyroid gland, but after a dose of 5 mg/kg in Sprague Dawley rats (both sexes) these organs showed Cmax similar to most other organs and tissues. There was no qualitative difference in distribution between male and female rats, but the female rats showed higher Cmax values in the intestinal tract. Protein binding differs between the species. The unbound fraction at concentrations of 1-10 uM is about 13% in human vs about 4% in rats and 8% in dogs. At the tested concentrations there was no effect of concentration or gender. In mice protein binding is much lower, with 44-6 % unbound, dependent on the tested concentration (range 100-2000 ng apixaban/mL). Plasma to blood ratios of about one in dog and human blood indicate uniform distribution between plasma and red blood cells and thus no specific distribution to red blood cells. For more Absorption, Distribution and Excretion (Complete) data for Apixaban (12 total), please visit the HSDB record page. Metabolism / Metabolites 50% of the orally administered dose is excreted as the unchanged parent compound, however 25% of the dose is excreted as O-demethyl apixaban sulfate. All apixaban metabolites account for approximately 32% of the excreted dose though the structure of all metabolites are not well defined. Apixaban is mainly metabolized by cytochrome p450(CYP)3A4 and to a lesser extent by CYP1A2, CYP2C8, CYP2C9, CYP2C19, and CYP2J2. Approximately 25% of an orally administered apixaban dose is recovered in urine and feces as metabolites. Apixaban is metabolized mainly via CYP3A4 with minor contributions from CYP1A2, 2C8, 2C9, 2C19, and 2J2. O-demethylation and hydroxylation at the 3-oxopiperidinyl moiety are the major sites of biotransformation. Unchanged apixaban is the major drug-related component in human plasma; there are no active circulating metabolites. Apixaban is mainly metabolized by CYP3A4/5 with conjugation via SULT1A1, but several other CYP and SULT isozymes are also involved. No apixaban metabolites were found to have pharmacological activity and there were no unique human metabolites. The metabolism and disposition of (14)C-apixaban, an orally bioavailable, highly selective, and direct acting/reversible factor Xa inhibitor, was investigated in 10 healthy male subjects without (group 1, n=6) and with bile collection (group 2, n=4) after a single 20-mg oral dose. Urine, blood, and feces samples were collected from all subjects. Bile samples were also collected for 3 to 8 hr after dosing from group 2 subjects. There were no serious adverse events or discontinuations due to adverse effects. In plasma, apixaban was the major circulating component and O-demethyl apixaban sulfate, a stable and water-soluble metabolite, was the significant metabolite. The exposure of apixaban (C(max) and area under the plasma concentration versus time curve) in subjects with bile collection was generally similar to that in subjects without bile collection. The administered dose was recovered in feces (group 1, 56.0%; group 2, 46.7%) and urine (group 1, 24.5%; group 2, 28.8%), with the parent drug representing approximately half of the recovered dose. Biliary excretion represented a minor elimination pathway (2.44% of the administered dose) from group 2 subjects within the limited collection period. Metabolic pathways identified for apixaban included O-demethylation, hydroxylation, and sulfation of hydroxylated O-demethyl apixaban. Thus, apixaban is an orally bioavailable inhibitor of factor Xa with elimination pathways that include metabolism and renal excretion. The metabolism and disposition of (14)C-apixaban, a potent, reversible, and direct inhibitor of coagulation factor Xa, were investigated in mice, rats, rabbits, dogs, and humans after a single oral administration and in incubations with hepatocytes. In plasma, the parent compound was the major circulating component in mice, rats, dogs, and humans. O-Demethyl apixaban sulfate (M1) represented approximately 25% of the parent area under the time curve in human plasma. This sulfate metabolite was present, but in lower amounts relative to the parent, in plasma from mice, rats, and dogs. Rabbits showed a plasma metabolite profile distinct from that of other species with apixaban as a minor component and M2 (O-demethyl apixaban) and M14 (O-demethyl apixaban glucuronide) as prominent components. The fecal route was a major elimination pathway, accounting for >54% of the dose in animals and >46% in humans. The urinary route accounted for <15% of the dose in animals and 25 to 28% in humans. Apixaban was the major component in feces of every species and in urine of all species except rabbit. M1 and M2 were common prominent metabolites in urine and feces of all species as well as in bile of rats and humans. In vivo metabolite profiles showed quantitative differences between species and from in vitro metabolite profiles, but all human metabolites were found in animal species. After intravenous administration of (14)C-apixaban to bile duct-cannulated rats, the significant portion (approximately 22%) of the dose was recovered as parent drug in the feces, suggesting direct excretion of the drug from gastrointestinal tracts of rats. Overall, apixaban was effectively eliminated via multiple elimination pathways in animals and humans, including oxidative metabolism, and direct renal and intestinal excretion. ... The O-demethyl apixaban sulfate is a major circulating metabolite in humans but circulates at lower concentrations relative to parent in animals. The aim of this study was to identify the sulfotransferases (SULTs) responsible for the sulfation reaction. Apixaban undergoes O-demethylation catalyzed by cytochrome P450 enzymes to O-demethyl apixaban, and then is conjugated by SULTs to form O-demethyl apixaban sulfate. Of the five human cDNA-expressed SULTs tested, SULT1A1 and SULT1A2 exhibited significant levels of catalytic activity for formation of O-demethyl apixaban sulfate, and SULT1A3, SULT1E1, and SULT2A1 showed much lower catalytic activities. In human liver S9, quercetin, a highly selective inhibitor of SULT1A1 and SULT1E1, inhibited O-demethyl apixaban sulfate formation by 99%; 2,6-dichloro-4-nitrophenol, another inhibitor of SULT1A1, also inhibited this reaction by >90%; estrone, a competitive inhibitor for SULT1E1, had no effect on this reaction. The comparable K(m) values for formation of O-demethyl apixaban sulfate were 41.4 microM (human liver S9), 36.8 microM (SULT1A1), and 70.8 microM (SULT1A2). Because of the high level of expression of SULT1A1 in liver and its higher level of catalytic activity for formation of O-demethyl apixaban sulfate, SULT1A1 might play a major role in humans for formation of O-demethyl apixaban sulfate. O-Demethyl apixaban was also investigated in liver S9 of mice, rats, rabbits, dogs, monkeys, and humans. The results indicated that liver S9 samples from dogs, monkeys, and humans had higher activities for formation of O-demethyl apixaban sulfate than those of mice, rats, and rabbits. Biological Half-Life 12.7±8.55h. Apixaban has ... an apparent half-life of approximately 12 hours following oral administration. In a comparative study elimination half-life in rats (2-3 hrs) was shorter than in dogs (5-6 hrs) and chimpanzees (5-7 hrs). Distribution volume is relatively low in rats (0.31 L/kg), dogs (0.30 L/kg) and chimpanzees (0.17 L/kg). ... After a single oral administration ... the elimination half life of radioactivity in blood was 1.7 to 4.2 hr. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Apixaban white to pale-yellow powder formulated into a film-coated tablet. Apixaban is indicated in patients to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. It is also indicated for the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE), in patients who have undergone hip or knee replacement surgery. It is also indicated for the treatment of PE and for the treatment of DVT. Finally, apixaban is indicated it patients to reduce the risk of recurrent DVT and PE following initial therapy. HUMAN EXPOSURE AND TOXICITY: Premature discontinuation of any oral anticoagulant, including apixaban, in the absence of adequate alternative anticoagulation increases the risk of thrombotic events. An increased rate of stroke was observed during the transition from apixaban to warfarin in clinical trials in atrial fibrillation patients. Apixaban increases the risk of hemorrhage and can cause serious, potentially fatal, bleeding. The drug should be discontinued if active pathological hemorrhage occurs. Epidural or spinal hematomas may occur in patients treated with apixaban who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include: use of indwelling epidural catheters concomitant use of other drugs that affect hemostasis, such as nonsteroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants a history of traumatic or repeated epidural or spinal punctures a history of spinal deformity or spinal surgery. Apixaban should not be used in people with mechanical heart valves. ANIMAL STUDIES: Single dose oral studies in mice (up to 4000 mg/kg), rats (up to 4510 mg/kg), dogs (up to 1500 mg/kg) and cynomolgus monkeys (up to 300 mg/kg) revealed no other drug-related effects than those related to the pharmacodynamic action of apixaban. In particular some monkeys died due to excessive bleeding after blood sampling. Apixaban was not carcinogenic when administered to mice and rats for up to 2 years. The systemic exposures (AUCs) of unbound apixaban in male and female mice at the highest doses tested (1500 and 3000 mg/kg/day) were 9 and 20 times, respectively, the human exposure of unbound drug at the MRHD of 10 mg/day. Systemic exposures of unbound apixaban in male and female rats at the highest dose tested (600 mg/kg/day) were 2 and 4 times, respectively, the human exposure. Apixaban had no effect on fertility in male or female rats when given at doses up to 600 mg/kg/day, a dose resulting in exposure levels that are 3 and 4 times, respectively, the human exposure. Apixaban administered to female rats at doses up to 1000 mg/kg/day from implantation through the end of lactation produced no adverse findings in male offspring (F1 generation) at doses up to 1000 mg/kg/day, a dose resulting in exposure that is 5 times the human exposure. Adverse effects in the F1-generation female offspring were limited to decreased mating and fertility indices at 1000 mg/kg/day. Apixaban was neither mutagenic in the bacterial reverse mutation (Ames) assay, nor clastogenic in Chinese hamster ovary cells in vitro, in a 1-month in vivo/in vitro cytogenetics study in rat peripheral blood lymphocytes, or in a rat micronucleus study in vivo. Hepatotoxicity Apixaban is associated with serum aminotransferase elevations greater than 3 times the upper limit of normal in 1% to 2% of treated patients. This rate is similar or lower than rates with warfarin or comparator arms. In premarketing studies, no instances of clinically apparent liver injury were reported, but subsequent to its approval and more wide scale use, several reports of mild but clinically apparent liver injury have been published. The liver injury arose within days of starting apixaban and the pattern of liver enzyme elevations was hepatocellular. Immunoallergic and autoimmune features were not present. In most cases, recovery was rapid once apixaban was stopped. In one analysis of a national health care database, the incidence of hospitalization for acute liver injury after initiation of apixaban therapy was 1 per 2,200 patients treated, a rate similar to that of rivaroxaban. Likelihood score: B (possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Information from four mothers indicates that apixaban levels in milk are rather high. An alternate drug is preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 92-94%. Interactions Current evidence suggests that addition of apixaban to standard antiplatelet therapy (e.g., aspirin, clopidogrel) does not substantially reduce the rate of recurrent ischemic events in patients with acute coronary syndrome (ACS) and may increase the risk of major, sometimes fatal, bleeding. /NOT included in US product label/ In a study in healthy individuals, concomitant administration of apixaban and enoxaparin had no effect on the pharmacokinetics of apixaban, but had an additive effect on anti-factor Xa activity. The increased pharmacodynamic effect was considered to be modest. When administration of apixaban and enoxaparin was separated by 6 hours in this study, the additive effect on anti-factor Xa activity was attenuated. In the principal efficacy study of apixaban, bleeding risk was increased in patients who received concomitant apixaban and aspirin therapy. Additionally, a placebo-controlled study of patients with acute coronary syndromes was terminated early after an increased risk of bleeding was observed in patients receiving apixaban in combination with aspirin and clopidogrel. In drug interaction studies in healthy individuals, apixaban did not substantially alter the pharmacokinetics of aspirin, and a pharmacodynamic interaction was not observed with such concomitant therapy. Concomitant use of apixaban and drugs that affect hemostasis (e.g., aspirin or other antiplatelet drugs, heparin or other anticoagulants, fibrinolytics, selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), nonsteroidal anti-inflammatory agents (NSAIAs)) increases the risk of bleeding. For more Interactions (Complete) data for Apixaban (11 total), please visit the HSDB record page. |
||
| 参考文献 |
|
||
| 其他信息 |
Therapeutic Uses
Eliquis (apixaban) is indicated to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. /Included in US product label/ Eliquis is indicated for the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE), in patients who have undergone hip or knee replacement surgery. /Included in US product label/ Eliquis is indicated for the treatment of pulmonary embolism (PE). /Included in US product label/ Eliquis is indicated for the treatment of deep vein thrombosis (DVT). /Included in US product label/ For more Therapeutic Uses (Complete) data for Apixaban (7 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ WARNING: PREMATURE DISCONTINUATION OF ELIQUIS INCREASES THE RISK OF THROMBOTIC EVENTS. Premature discontinuation of any oral anticoagulant, including Eliquis, increases the risk of thrombotic events. If anticoagulation with Eliquis is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant. /BOXED WARNING/ WARNING: SPINAL/EPIDURAL HEMATOMA. Epidural or spinal hematomas may occur in patients treated with Eliquis who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include: use of indwelling epidural catheters concomitant use of other drugs that affect hemostasis, such as nonsteroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants a history of traumatic or repeated epidural or spinal punctures a history of spinal deformity or spinal surgery optimal timing between the administration of Eliquis and neuraxial procedures is not known. Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary. Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated. FDA Pregnancy Risk Category: B /NO EVIDENCE OF RISK IN HUMANS. Adequate, well controlled studies in pregnant women have not shown increased risk of fetal abnormalities despite adverse findings in animals, or, in the absence of adequate human studies, animal studies show no fetal risk. The chance of fetal harm is remote but remains a possibility./ It is unknown whether apixaban or its metabolites are excreted in human milk. Rats excrete apixaban in milk (12% of the maternal dose). Women should be instructed either to discontinue breastfeeding or to discontinue Eliquis therapy, taking into account the importance of the drug to the mother. For more Drug Warnings (Complete) data for Apixaban (17 total), please visit the HSDB record page. Pharmacodynamics Apixaban selectively inhibits factor Xa in its free and bound forms, independant of antithrombin III. Apixaban also inhibits prothrominase. These effects prevent the formation of a thrombus. Apixaban is a pyrazolopyridine that is 7-oxo-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide substituted at position 1 by a 4-methoxyphenyl group and at position 6 by a 4-(2-oxopiperidin-1-yl)phenyl group. It is used for the prevention and treatment of thromboembolic diseases. It has a role as an anticoagulant and an EC 3.4.21.6 (coagulation factor Xa) inhibitor. It is a pyrazolopyridine, a member of piperidones, a lactam and an aromatic ether. Apixaban is an oral, direct, and highly selective factor Xa (FXa) inhibitor of both free and bound FXa, as well as prothrombinase, independent of antithrombin III for the prevention and treatment of thromboembolic diseases. It is marketed under the name Eliquis. Apixaban was approved by the FDA on December 28, 2012. Apixaban is a Factor Xa Inhibitor. The mechanism of action of apixaban is as a Factor Xa Inhibitor. Apixaban is an oral anticoagulant and direct inhibitor of factor Xa which is used to decrease the risk of venous thromboses, systemic embolization and stroke in patients with atrial fibrillation, and lower the risk of deep vein thrombosis and pulmonary embolus after knee or hip replacement surgery. Apixaban has been linked to a low rate of serum aminotransferase elevations during therapy and to rare instances of clinically apparent liver injury. Apixaban is an orally active inhibitor of coagulation factor Xa with anticoagulant activity. Apixaban directly inhibits factor Xa, thereby interfering with the conversion of prothrombin to thrombin and preventing formation of cross-linked fibrin clots. APIXABAN is a small molecule drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 2011 and has 8 approved and 20 investigational indications. This drug has a black box warning from the FDA. Efforts to identify a suitable follow-on compound to razaxaban (compound 4) focused on modification of the carboxamido linker to eliminate potential in vivo hydrolysis to a primary aniline. Cyclization of the carboxamido linker to the novel bicyclic tetrahydropyrazolopyridinone scaffold retained the potent fXa binding activity. Exceptional potency of the series prompted an investigation of the neutral P1 moieties that resulted in the identification of the p-methoxyphenyl P1, which retained factor Xa binding affinity and good oral bioavailability. Further optimization of the C-3 pyrazole position and replacement of the terminal P4 ring with a neutral heterocycle culminated in the discovery of 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide (apixaban, compound 40). Compound 40 exhibits a high degree of fXa potency, selectivity, and efficacy and has an improved pharmacokinetic profile relative to 4.[1] Background: Apixaban is an oral, direct and highly selective factor Xa (FXa) inhibitor in late-stage clinical development for the prevention and treatment of thromboembolic diseases. Objective: We evaluated the in vitro properties of apixaban and its in vivo activities in rabbit models of thrombosis and hemostasis. Methods: Studies were conducted in arteriovenous-shunt thrombosis (AVST), venous thrombosis (VT), electrically mediated carotid arterial thrombosis (ECAT) and cuticle bleeding time (BT) models. Results: In vitro, apixaban is potent and selective, with a K(i) of 0.08 nm for human FXa. It exhibited species difference in FXa inhibition [FXa K(i) (nm): 0.16, rabbit; 1.3, rat; 1.7, dog] and anticoagulation [EC(2x) (microm, concentration required to double the prothrombin time): 3.6, human; 2.3, rabbit; 7.9, rat; 6.7, dog]. Apixaban at 10 microm did not alter human and rabbit platelet aggregation to ADP, gamma-thrombin, and collagen. In vivo, the values for antithrombotic ED(50) (dose that reduced thrombus weight or increased blood flow by 50% of the control) in AVST, VT and ECAT and the values for BT ED(3x) (dose that increased BT by 3-fold) were 0.27 +/- 0.03, 0.11 +/- 0.03, 0.07 +/- 0.02 and > 3 mg kg(-1) h(-1) i.v. for apixaban, 0.05 +/- 0.01, 0.05 +/- 0.01, 0.27 +/- 0.08 and > 3 mg kg(-1) h(-1) i.v. for the indirect FXa inhibitor fondaparinux, and 0.53 +/- 0.04, 0.27 +/- 0.01, 0.08 +/- 0.01 and 0.70 +/- 0.07 mg kg(-1) day(-1) p.o. for the oral anticoagulant warfarin, respectively.[2] In summary, apixaban was as effective as the current anticoagulant standard of care in the prevention of thrombosis at doses that preserve hemostasis in rabbits. HepTest, mPT and chromogenic anti‐FXa assays were potential markers to monitor the anticoagulant and plasma levels of apixaban. Because of the favorable preclinical profile of apixaban, this compound was selected for clinical development. Similar to its favorable preclinical findings, initial phase II studies with apixaban have demonstrated its efficacy and safety in the prevention and treatment of venous thromboembolism. Other potential indications for apixaban in the treatment and prevention of various life‐threatening thromboembolic events are currently being elucidated in the clinic.[2] |
| 分子式 |
C25H25N5O4
|
|---|---|
| 分子量 |
459.5
|
| 精确质量 |
459.19
|
| 元素分析 |
C, 65.35; H, 5.48; N, 15.24; O, 13.93
|
| CAS号 |
503612-47-3
|
| 相关CAS号 |
Apixaban-13C,d3;1261393-15-0;Apixaban-d3;1131996-12-7
|
| PubChem CID |
10182969
|
| 外观&性状 |
Off-white to yellow solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
770.5±60.0 °C at 760 mmHg
|
| 闪点 |
419.8±32.9 °C
|
| 蒸汽压 |
0.0±2.6 mmHg at 25°C
|
| 折射率 |
1.705
|
| LogP |
0.48
|
| tPSA |
110.76
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
777
|
| 定义原子立体中心数目 |
0
|
| SMILES |
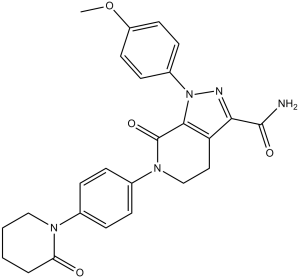
COC1=CC=C(C=C1)N2C3=C(CCN(C3=O)C4=CC=C(C=C4)N5CCCCC5=O)C(=N2)C(=O)N
|
| InChi Key |
QNZCBYKSOIHPEH-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32)
|
| 化学名 |
1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5-dihydropyrazolo[5,4-c]pyridine-3-carboxamide
|
| 别名 |
BMS56224701; BMS 56224701; Apixaban; 503612-47-3; Eliquis; BMS-562,247-01; BMS-562,247; BMS 562,247-01; 1-(4-Methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide; apixabanum; BMS 562247-01; Apixaban, BMS-56224701; brand name: Eliquis
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.44 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.44 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 30% PEG400+0.5% Tween80+5% propylene glycol:30 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1763 mL | 10.8814 mL | 21.7628 mL | |
| 5 mM | 0.4353 mL | 2.1763 mL | 4.3526 mL | |
| 10 mM | 0.2176 mL | 1.0881 mL | 2.1763 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04191928 | Completed | Drug: Apixaban | Pancreas Cancer DVT |
Thomas Jefferson University | March 3, 2020 | Phase 1 |
| NCT04344717 | Recruiting | Drug: Apixaban single dose Drug: Apixaban steady-state |
Short Bowel Syndrome Anticoagulation |
Universitaire Ziekenhuizen KU Leuven | December 20, 2020 | Phase 4 |
| NCT04952792 | Completed | Drug: Apixaban 2.5 milligram Oral Tablet |
Atrial Fibrillation Hemodialysis Complication |
Hospital Universitari de Bellvitge | May 20, 2021 | Phase 2 |
| NCT05632445 | Completed | Drug: Apixaban vs. DAPT | Left Atrial Appendage Occlusion | DR. XAVIER FREIXA | May 1, 2019 | Phase 4 |
Plot of apixaban inhibition of human FXa activity at different concentrations of the chromogenic peptide substrate S‐2765.J Thromb Haemost.2008 May;6(5):820-9. |
|---|
Antithrombotic effects in the arteriovenous‐shunt thrombosis (AVST) and venous thrombosis (VT) rabbit models.J Thromb Haemost.2008 May;6(5):820-9. |
Antithrombotic effects in the rabbit model of electrically mediated carotid arterial thrombosis.J Thromb Haemost.2008 May;6(5):820-9. |