| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
艾多沙班以浓度依赖性方式延长人血浆的 PT、TT 和 APTT(分别为 1、1 和 5 分钟)[1]。艾多沙班的 IC50 为 2.90 µM,可防止凝血酶引起的血小板聚集[1]。
|
|---|---|
| 体内研究 (In Vivo) |
艾多沙班可延长 PT,并在 0.5、2.5 和 12.5 mg/kg 剂量下显着且剂量依赖性地减少血栓形成;宝;一次[1]。
|
| 细胞实验 |
细胞活力测定[1]
细胞类型:人、大鼠、食蟹猴和兔血浆;人血小板 测试浓度: 孵育时间: 1 和 5 分钟 实验结果: 抗凝血酶。 |
| 动物实验 |
Animal/Disease Models: Male Slc: Wistar rats (210-240 g); Male New Zealand White rabbits(2.5-3.5 kg) (Both are venous stasis thrombosis model)[1].
Doses: 0.5, 2.5 and 12.5 mg/kg Route of Administration: Oral administration; once Experimental Results: Inhibited exogenous FXa activity. Antithrombotic. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following oral administration, peak plasma edoxaban concentrations are observed within 1-2 hours. Absolute bioavailability is 62%. Edoxaban is eliminated primarily as unchanged drug in urine. Renal clearance (11 L/hour) accounts for approximately 50% of the total clearance of edoxaban (22 L/hour). Metabolism and biliary/intestinal excretion account for the remaining clearance. The steady state volume of distribution is 107 L. 22 L/hr /MILK/ There are no data on the presence of edoxaban in human milk ... . Edoxaban was present in rat milk. ... Disposition is biphasic. The steady-state volume of distribution (Vdss) is 107 (19.9) L (mean (SD)). In vitro plasma protein binding is approximately 55%. There is no clinically relevant accumulation of edoxaban (accumulation ratio 1.14) with once daily dosing. Administration of a crushed 60 mg tablet, either mixed into applesauce or suspended in water and given through a nasogastric tube, showed similar exposure compared to administration of an intact tablet. Edoxaban is eliminated primarily as unchanged drug in the urine. Renal clearance (11 L/hour) accounts for approximately 50% of the total clearance of edoxaban (22 L/hour). Metabolism and biliary/intestinal excretion account for the remaining clearance. Following oral administration, peak plasma edoxaban concentrations are observed within 1-2 hours. Absolute bioavailability is 62%. Food does not affect total systemic exposure to edoxaban. Savaysa was administered with or without food in the ENGAGE AF-TIMI 48 and Hokusai VTE trials. Metabolism / Metabolites Edoxaban is not extensively metabolized by CYP3A4 resulting in minimal drug-drug interactions. However, it does interact with drugs that inhibit p-gp (p-glycoprotein), which is used to transport edoxaban across the intestinal wall. Unchanged edoxaban is the predominant form in plasma. There is minimal metabolism via hydrolysis (mediated by carboxylesterase 1), conjugation, and oxidation by CYP3A4. The predominant metabolite M-4, formed by hydrolysis, is human-specific and active and reaches less than 10% of the exposure of the parent compound in healthy subjects. Exposure to the other metabolites is less than 5% of exposure to edoxaban. ... All subjects received a single oral 60 mg edoxaban dose in period 1, and 7 days of 600 mg rifampin (2 x 300 mg capsules once daily) with a single oral edoxaban 60 mg dose administered concomitantly on day 7 in period 2. A 6-day washout period separated the treatments. Plasma concentrations of edoxaban and its metabolites M4 and M6 were measured, and limited assessments of pharmacodynamic markers of coagulation were performed. In total, 34 healthy subjects were enrolled; 32 completed the study. Coadministration of rifampin with edoxaban decreased edoxaban exposure but increased active metabolite exposure. Rifampin increased apparent oral clearance of edoxaban by 33% and decreased its half-life by 50%. Anticoagulant effects based on the prothrombin time (PT) and the activated partial thromboplastin time (aPTT) with and without rifampin at early time points were maintained to a greater-than-expected degree than with edoxaban exposure alone, presumably because of an increased contribution from the active metabolites. Edoxaban was well tolerated in this healthy adult population. Rifampin reduced exposure to edoxaban while increasing exposure to its active metabolites M4 and M6. PT and aPTT at early time points did not change appreciably; however, the data should be interpreted with caution. Edoxaban and its low-abundance, active metabolite M4 are substrates of P-glycoprotein (P-gp; MDR1) and organic anion transporter protein 1B1 (OATP1B1), respectively, and pharmacological inhibitors of P-gp and OATP1B1 can affect edoxaban and M4 pharmacokinetics (PK). In this integrated pharmacogenomic analysis, genotype and concentration-time data from 458 healthy volunteers in 14 completed phase 1 studies were pooled to examine the impact on edoxaban PK parameters of allelic variants of ABCB1 (rs1045642: C3435T) and SLCO1B1 (rs4149056: T521C), which encode for P-gp and OATP1B1. Although some pharmacologic inhibitors of P-gp and OATP1B1 increase edoxaban exposure, neither the ABCB1 C3435T nor the SLCO1B1 T521C polymorphism affected edoxaban PK. A slight elevation in M4 exposure was observed among SLCO1B1 C-allele carriers; however, this elevation is unlikely to be clinically significant as plasma M4 concentrations comprise <10% of total edoxaban levels. The predominant metabolite M-4, formed by hydrolysis, is human-specific and active and reaches less than 10% of the exposure of the parent compound in healthy subjects. Exposure to the other metabolites is less than 5% of exposure to edoxaban. Unchanged edoxaban is the predominant form in plasma. There is minimal metabolism via hydrolysis (mediated by carboxylesterase 1), conjugation, and oxidation by CYP3A4. Biological Half-Life The terminal elimination half-life of edoxaban following oral administration is 10 to 14 hours. The terminal elimination half-life of edoxaban following oral administration is 10 to 14 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Edoxaban is a white to pale yellowish-white crystalline powder. It is used to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. It is also used for the treatment of deep vein thrombosis (DVT) and pulmonary embolism following 5 to 10 days of initial therapy with a parenteral anticoagulant. HUMAN STUDIES: Overdose of the drug increases the risk of bleeding. Edoxaban increases the risk of hemorrhage and can cause serious, potentially fatal, bleeding. Patients should be promptly evaluated if any manifestations of blood loss occur during therapy. The drug should be discontinued if active pathological bleeding occurs. However, minor or "nuisance" bleeding is a common occurrence in patients receiving any anticoagulant and should not readily lead to treatment discontinuance. Edoxaban and its human-specific metabolite, M-4 were not genotoxic in in vitro human lymphocytes micronucleus test. ANIMAL STUDIES: Edoxaban was not carcinogenic when administered daily to mice and rats by oral gavage for up to 104 weeks. Edoxaban showed no effects on fertility and early embryonic development in rats at doses of up to 1000 mg/kg/day. In a rat pre- and post-natal developmental study, edoxaban was administered orally during the period of organogenesis and through lactation day 20 at doses up to 30 mg/kg/day. Vaginal bleeding in pregnant rats and delayed avoidance response (a learning test) in female offspring were seen at 30 mg/kg/day. Embryo-fetal development studies were conducted in pregnant rats and rabbits during the period of organogenesis. In rats, no malformation was seen when edoxaban was administered orally at doses up to 300 mg/kg/day. Increased post-implantation loss occurred at 300 mg/kg/day, but this effect may be secondary to the maternal vaginal hemorrhage seen at this dose in rats. In rabbits, no malformation was seen at doses up to 600 mg/kg/day. Embryo-fetal toxicities occurred at maternally toxic doses, and included absent or small fetal gallbladder at 600 mg/kg/day, and increased post-implantation loss, increased spontaneous abortion, and decreased live fetuses and fetal weight at doses equal to or greater than 200 mg/kg/day. Edoxaban and its human-specific metabolite, M-4, were genotoxic in in vitro chromosomal aberration tests but were not genotoxic in the in vitro bacterial reverse mutation (Ames test), in in vivo rat bone marrow micronucleus test, in in vivo rat liver micronucleus test, and in in vivo unscheduled DNA synthesis tests. Hepatotoxicity Edoxaban is associated with serum aminotransferase elevations greater than 3 times the upper limit of normal in 2% to 5% of treated patients. This rate is similar or lower than rates with warfarin or comparator arms. The elevations are generally transient and not associated with symptoms or jaundice. In premarketing studies, no instances of clinically apparent liver injury were reported, but there was little experience in large numbers of patients treated for extend periods of time. In large health care databases, the rate of liver injury has been somewhat less with edoxaban than rivaroxaban and apixaban, but the numbers of patients treated with edoxaban has been limited and the nature of the liver injury not described. Likelihood score: D (possible race cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because no information is available on the use of edoxaban during breastfeeding and the drug is orally absorbable, an alternate drug is preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding In vitro plasma protein binding is ~55%. Interactions Edoxaban, an oral direct factor Xa inhibitor, is in development for thromboprophylaxis, including prevention of stroke and systemic embolism in patients with atrial fibrillation (AF). P-glycoprotein (P-gp), an efflux transporter, modulates absorption and excretion of xenobiotics. Edoxaban is a P-gp substrate, and several cardiovascular (CV) drugs have the potential to inhibit P-gp and increase drug exposure. /The objective of the study was/ to assess the potential pharmacokinetic interactions of edoxaban and 6 cardiovascular drugs used in the management of AF and known P-gp substrates/inhibitors. Drug-drug interaction studies with edoxaban and CV drugs with known P-gp substrate/inhibitor potential were conducted in healthy subjects. In 4 crossover, 2-period, 2-treatment studies, subjects received edoxaban 60 mg alone and coadministered with quinidine 300 mg (n = 42), verapamil 240 mg (n = 34), atorvastatin 80 mg (n = 32), or dronedarone 400 mg (n = 34). Additionally, edoxaban 60 mg alone and coadministered with amiodarone 400 mg (n = 30) or digoxin 0.25 mg (n = 48) was evaluated in a single-sequence study and 2-cohort study, respectively. Edoxaban exposure measured as area under the curve increased for concomitant administration of edoxaban with quinidine (76.7%), verapamil (52.7%), amiodarone (39.8%), and dronedarone (84.5%), and exposure measured as 24 hr concentrations for quinidine (11.8%), verapamil (29.1%), and dronedarone (157.6%) also increased. Administration of edoxaban with amiodarone decreased the 24-hr concentration for edoxaban by 25.7%. Concomitant administration with digoxin or atorvastatin had minimal effects on edoxaban exposure. Coadministration of the P-gp inhibitors quinidine, verapamil, and dronedarone increased edoxaban exposure. Modest/minimal effects were observed for amiodarone, atorvastatin, and digoxin. The oral direct factor Xa inhibitor edoxaban is a P-glycoprotein (P-gp) substrate metabolized via carboxylesterase-1 and cytochrome P450 (CYP) 3A4/5. The effect of rifampin-induced induction of P-gp and CYP3A4/5 on transport and metabolism of edoxaban through the CYP3A4/5 pathway was investigated in a single-dose edoxaban study. This was a phase 1, open-label, two-treatment, two-period, single-sequence drug interaction study in healthy adults. All subjects received a single oral 60 mg edoxaban dose in period 1, and 7 days of 600 mg rifampin (2 x 300 mg capsules once daily) with a single oral edoxaban 60 mg dose administered concomitantly on day 7 in period 2. A 6-day washout period separated the treatments. Plasma concentrations of edoxaban and its metabolites M4 and M6 were measured, and limited assessments of pharmacodynamic markers of coagulation were performed. In total, 34 healthy subjects were enrolled; 32 completed the study. Coadministration of rifampin with edoxaban decreased edoxaban exposure but increased active metabolite exposure. Rifampin increased apparent oral clearance of edoxaban by 33% and decreased its half-life by 50%. Anticoagulant effects based on the prothrombin time (PT) and the activated partial thromboplastin time (aPTT) with and without rifampin at early time points were maintained to a greater-than-expected degree than with edoxaban exposure alone, presumably because of an increased contribution from the active metabolites. Edoxaban was well tolerated in this healthy adult population. Rifampin reduced exposure to edoxaban while increasing exposure to its active metabolites M4 and M6. PT and aPTT at early time points did not change appreciably; however, the data should be interpreted with caution. Verapamil increased peak plasma concentrations and systemic exposure of edoxaban by approximately 53%; pharmacokinetic parameters of verapamil were altered to only a slight extent. Dosage of edoxaban should be reduced when the drug is administered concomitantly with verapamil in patients with venous thromboembolism. Quinidine increased peak plasma concentrations and systemic exposure of edoxaban by approximately 85 and 77%, respectively, but edoxaban did not affect pharmacokinetics of quinidine. Dosage of edoxaban should be reduced when the drug is administered concomitantly with quinidine in patients with venous thromboembolism. For more Interactions (Complete) data for Edoxaban (19 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Factor Xa Inhibitors /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Edoxaban is included in the database. Savaysa is indicated to reduce the risk of stroke and systemic embolism (SE) in patients with nonvalvular atrial fibrillation (NVAF). /Included in US product label/ Savaysa is indicated for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) following 5 to 10 days of initial therapy with a parenteral anticoagulant. /Included in US product label/ For more Therapeutic Uses (Complete) data for Edoxaban (7 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ REDUCED EFFICACY IN NONVALVULAR ATRIAL FIBRILLATION PATIENTS WITH CRCL > 95 ML/MIN. Savaysa should not be used in patients with CrCL > 95 mL/min. In the ENGAGE AF-TIMI 48 study, nonvalvular atrial fibrillation patients with CrCL > 95 mL/min had an increased rate of ischemic stroke with Savaysa 60 mg once daily compared to patients treated with warfarin. In these patients another anticoagulant should be used. /BOXED WARNING/ PREMATURE DISCONTINUATION OF SAVAYSA INCREASES THE RISK OF ISCHEMIC EVENTS. Premature discontinuation of any oral anticoagulant in the absence of adequate alternative anticoagulation increases the risk of ischemic events. If Savaysa is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant as described in the transition guidance. /BOXED WARNING/ SPINAL/EPIDURAL HEMATOMA. Epidural or spinal hematomas may occur in patients treated with Savaysa who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include: use of indwelling epidural catheters; concomitant use of other drugs that affect hemostasis, such as nonsteroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants; a history of traumatic or repeated epidural or spinal punctures; a history of spinal deformity or spinal surgery; optimal timing between the administration of Savaysa and neuraxial procedures is not known. Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessar. Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated. Safety and efficacy of edoxaban have not been evaluated in patients with mechanical heart valves or moderate to severe mitral stenosis; use of the drug is not recommended in such patients. For more Drug Warnings (Complete) data for Edoxaban (18 total), please visit the HSDB record page. Pharmacodynamics Administration of edoxaban results in prolongation of clotting time tests such as aPTT (activated partial thromboplastin time), PT (prothrombin time), and INR (international normalized ratio). |
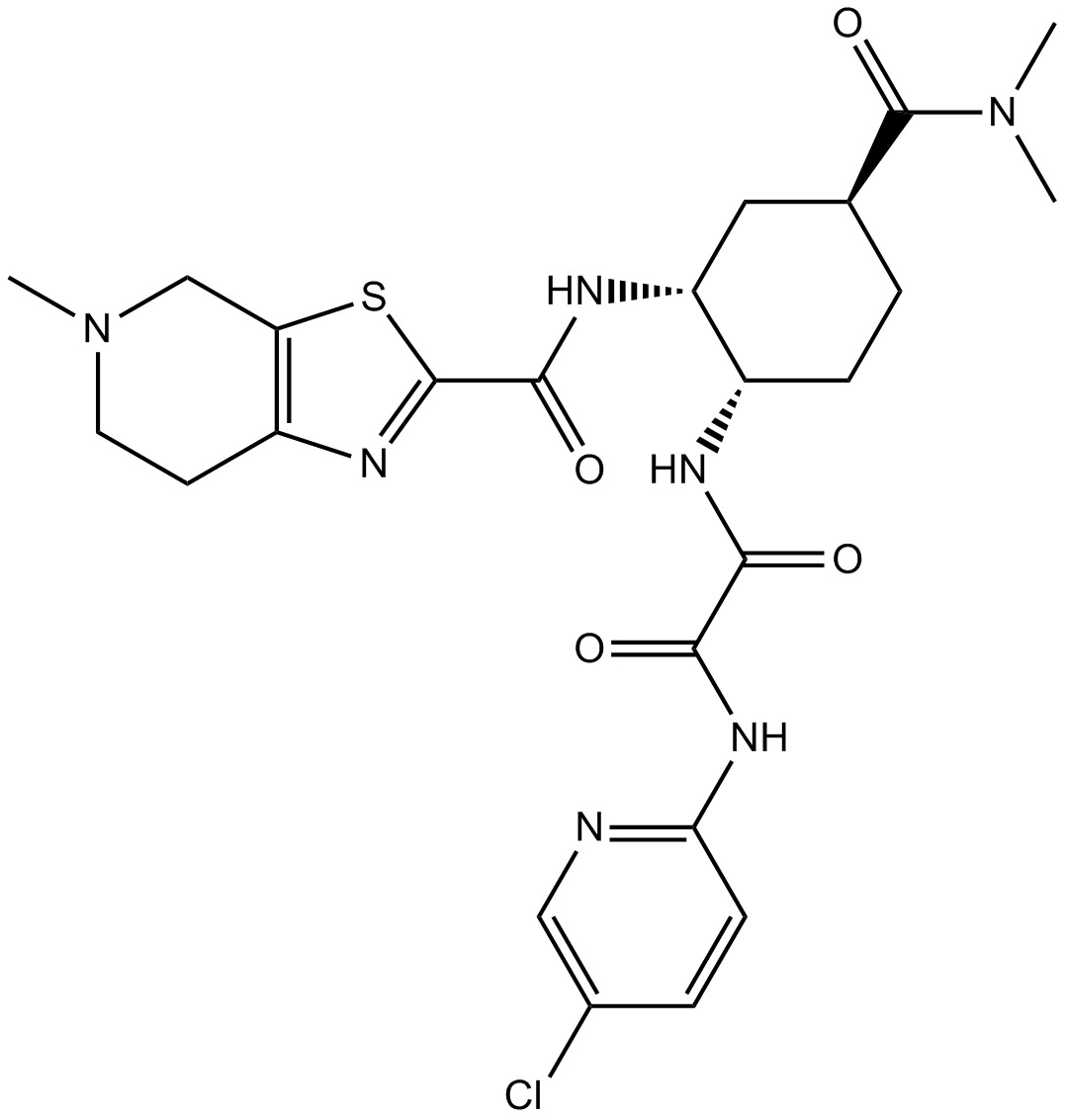
| 分子式 |
C24H30CLN7O4S
|
|
|---|---|---|
| 分子量 |
548.06
|
|
| 精确质量 |
547.176
|
|
| CAS号 |
480449-70-5
|
|
| 相关CAS号 |
Edoxaban tosylate;480449-71-6;Edoxaban tosylate monohydrate;1229194-11-9;Edoxaban-d6;1304701-57-2;Edoxaban hydrochloride;480448-29-1
|
|
| PubChem CID |
10280735
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 熔点 |
Crystals as monohydrate from ethanol + water. MP: 245-48 (decomposes) /Edoxaban tosylate/
|
|
| 折射率 |
1.646
|
|
| LogP |
1.24
|
|
| tPSA |
164.87
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
8
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
37
|
|
| 分子复杂度/Complexity |
880
|
|
| 定义原子立体中心数目 |
3
|
|
| SMILES |
CN1CCC2=C(C1)SC(=N2)C(=O)N[C@@H]3C[C@H](CC[C@@H]3NC(=O)C(=O)NC4=NC=C(C=C4)Cl)C(=O)N(C)C
|
|
| InChi Key |
HGVDHZBSSITLCT-JLJPHGGASA-N
|
|
| InChi Code |
InChI=1S/C24H30ClN7O4S/c1-31(2)24(36)13-4-6-15(27-20(33)21(34)30-19-7-5-14(25)11-26-19)17(10-13)28-22(35)23-29-16-8-9-32(3)12-18(16)37-23/h5,7,11,13,15,17H,4,6,8-10,12H2,1-3H3,(H,27,33)(H,28,35)(H,26,30,34)/t13-,15-,17+/m0/s1
|
|
| 化学名 |
N'-(5-chloropyridin-2-yl)-N-[(1S,2R,4S)-4-(dimethylcarbamoyl)-2-[(5-methyl-6,7-dihydro-4H-[1,3]thiazolo[5,4-c]pyridine-2-carbonyl)amino]cyclohexyl]oxamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8246 mL | 9.1231 mL | 18.2462 mL | |
| 5 mM | 0.3649 mL | 1.8246 mL | 3.6492 mL | |
| 10 mM | 0.1825 mL | 0.9123 mL | 1.8246 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Prospective Comparison of Incidence of Heavy Menstrual Bleeding in Women Treated With Direct Oral Anticoagulants
CTID: NCT04477837
Phase: Status: Completed
Date: 2024-08-21