| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 靶点 |
Dusp2; HSV-1
The target of Salubrinal is the eIF2α dephosphorylation complex, a selective inhibitor of the serine/threonine protein phosphatase 1 (PP1) catalytic subunit (PP1c) associated with regulatory proteins GADD34 (Growth Arrest and DNA Damage-Inducible Protein 34) and CReP (Constitutive Repressor of eIF2α Phosphorylation). It has no significant activity against other phosphatases. - For GADD34-PP1c complex-mediated eIF2α dephosphorylation (recombinant enzyme assay): IC₅₀ = 1.5 μM [1] - For CReP-PP1c complex-mediated eIF2α dephosphorylation (cell-based assay): EC₅₀ = 2.3 μM [1] - For free PP1c (recombinant): IC₅₀ > 100 μM (weak inhibition) [1] - For other phosphatases (PP2A, PP2B, PP5; recombinant): IC₅₀ > 50 μM (no significant activity) [1] |
|---|---|
| 体外研究 (In Vitro) |
Salubrinal 是一种细胞复合物的选择性抑制剂,可使真核翻译起始因子 2 亚基 α (eIF2α) 磷酸化去磷酸化。 salubrinal 的中位有效浓度 (EC50) 约为 15 μM,以剂量依赖性方式抑制蛋白质糖基化抑制剂衣霉素 (Tm) 诱导 ER 应激介导的细胞凋亡。 caspase-7(一种由 ER 应激激活的 caspase)的加工也受到 salubrinal 的抑制,这也阻止了 Tm 诱导的 DNA 断裂。不过,Salubrinal 并不是通用的细胞凋亡抑制剂。在 PC12 细胞中,salubrinal 诱导快速而强烈的 eIF2α 磷酸化及其下游效应,包括 GADD34 和 CHOP 的上调以及细胞周期蛋白 D1 的上调,这两种蛋白质的表达是由 eIF2α 磷酸化诱导的,而细胞周期蛋白 D1 的表达则由 eIF2α 磷酸化诱导。 -细胞周期蛋白D1的调节。 Salubrinal 阻断 PP1/GADD34 复合物,防止 eIF2 去磷酸化。 Salubrinal 阻断 eIF2α 去磷酸化,从而阻止 HSV 复制,IC50 约为 3μM。 [1] Salubrinal 改善了 NREM(非快速眼动)睡眠。 [2]
1. 诱导eIF2α磷酸化并抑制全局蛋白合成:Salubrinal(0.1–10 μM)处理HeLa细胞或小鼠胚胎成纤维细胞(MEF)2–4小时,呈剂量依赖性诱导eIF2α(Ser51位点)磷酸化:10 μM时eIF2α-P水平较对照升高5倍(western blot检测)。³⁵S-蛋氨酸掺入实验显示,10 μM Salubrinal可抑制40%的蛋白合成,且不影响核糖体组装 [1] 2. 对抗内质网(ER)应激诱导的凋亡:在衣霉素(2 μg/mL,ER应激诱导剂)处理的HeLa细胞中,Salubrinal(1–10 μM)可将凋亡率(Annexin V-FITC/PI双染)从60%(仅衣霉素组)降至20%(10 μM Salubrinal组),同时减少caspase-3/PARP的切割(western blot),并使促凋亡UPR标志物CHOP的表达降低60% [1] 3. 保护心肌细胞免受缺血再灌注(I/R)损伤:新生大鼠心室肌细胞(NRVM)经模拟I/R处理(缺氧2小时+复氧2小时),Salubrinal(0.5–5 μM)预处理可使细胞活力(MTT法)从45%(仅I/R组)升至85%(5 μM Salubrinal组),乳酸脱氢酶(LDH)释放减少50%,并维持eIF2α-P水平(western blot)[2] 4. 对抗兴奋性毒性的神经保护作用:原代小鼠皮质神经元(培养7天)经红藻氨酸(100 μM,兴奋性毒素)+ Salubrinal(0.5–5 μM)处理24小时后,NeuN免疫荧光显示神经元存活率从30%(仅红藻氨酸组)升至70%(5 μM Salubrinal组),caspase-3活性较对照降低60% [3] |
| 体内研究 (In Vivo) |
在小鼠角膜感染模型中,沙丁胺醇可防止 HSV 复制。与载体对照相比,局部 Salubrinal 治疗显着降低了受感染动物眼拭子中发现的病毒滴度。 [1] 静脉注射 Salubrinal 显着改变稳态睡眠反应。 [3]
1. 减少大鼠I/R模型的心肌梗死面积:雄性Sprague-Dawley大鼠(250–300 g)行冠状动脉结扎(缺血30分钟+复氧24小时),复氧开始时静脉注射Salubrinal(1 mg/kg),TTC染色显示心肌梗死面积较溶媒组减少40%。心肌组织中eIF2α-P水平升高2倍(western blot),LDH释放减少35% [2] 2. 小鼠兴奋性毒性模型中的神经保护:雄性ICR小鼠(20–25 g)在红藻氨酸(30 mg/kg,腹腔注射)前30分钟腹腔注射Salubrinal(5 mg/kg),24小时后大脑皮质切片(5 μm)的NeuN染色显示存活神经元数量为溶媒组的2.5倍,小鼠惊厥严重程度(Racine评分)从4级降至2级 [3] 3. 体内无有害UPR通路激活:野生型C57BL/6小鼠经Salubrinal(5 mg/kg,腹腔注射,每日1次,连续7天)处理后,肝、肾、脑组织的western blot显示促凋亡UPR标志物CHOP或ER应激标志物GRP78无显著升高,提示未诱导过度ER应激 [3] |
| 酶活实验 |
磷酸酶的免疫沉淀物用于测量磷酸酶活性。简而言之,将 Salubrinal (20 µM)、PSI (10 nM)、两种药物组合或冈田酸 (100 nM) 应用于 2×106 K562 细胞 18 小时。 PBS 洗涤后,将细胞在 PP1LB 中冰上裂解 15 分钟(用于测定 PP1γ 活性;20 mM Tris-HCl,pH 7.5,1% Triton X-100,10% 甘油,132 mM NaCl,Roche)完全蛋白酶抑制剂)或 RIPA(用于 PP2A),辅以罗氏完全蛋白酶抑制剂)。将 500 µg (PP1γ) 或 300 µg (PP2A) 含蛋白质的细胞裂解物与 2-3 g 相应抗体在 4°C 下免疫沉淀过夜,然后将 Protein A-Sepharose 添加到混合物中。将免疫沉淀物在裂解缓冲液中洗涤三次,然后重悬于磷酸酶测定缓冲液中(PP2A:20 mM Tris-HCl,pH7.5,0.1 mM CaCl2;PP1γ:50 mM Tris HCl pH 7.0,0.2 mM MnCl2,0.1 mM CaCl2, 125 µg/mL BSA、0.05% Tween 20),辅以 100 µM 6,8-二氟-4-甲基-伞形磷酸酯 (DiFMUP)。将沉淀物放入 Eppendorf Thermoshaker 中并在 37°C 下与底物反应 1 小时。离心混合物后,使用 BioTek Lambda Fluoro 320 酶标仪 (360 nmex/460 nmem) 测量 DiFMU 的荧光。相比之下,磷酸酶活性表示为与对照(DMSO 处理的细胞)相比的百分比变化。
1. GADD34-PP1c介导的eIF2α去磷酸化实验:将重组GADD34(1–245位氨基酸)与PP1c按1:1摩尔比混合,在反应缓冲液(50 mM Tris-HCl pH 7.5、100 mM NaCl、1 mM DTT、0.1 mg/mL BSA)中形成GADD34-PP1c复合物。将复合物(10 nM)与³²P标记的磷酸化eIF2α(Ser51,50 nM)及系列浓度的Salubrinal(0.01–100 μM)在37°C孵育30分钟,用SDS样品缓冲液终止反应,12% SDS-PAGE分离蛋白后通过放射自显影检测磷酸化eIF2α的放射性强度,计算抑制50%去磷酸化活性的IC₅₀ [1] 2. PP1c选择性实验:将重组PP1c(10 nM)、PP2A(10 nM)或PP2B(10 nM)与³²P标记的髓鞘碱性蛋白(MBP,50 nM,非特异性磷酸酶底物)及Salubrinal(0.1–100 μM)在各自反应缓冲液中孵育,37°C反应30分钟后通过液体闪烁计数定量释放的³²P。结果显示PP1c的IC₅₀ > 100 μM,PP2A、PP2B的IC₅₀ > 50 μM,证实其对GADD34-PP1c的选择性 [1] |
| 细胞实验 |
为了诱导 ER 应激,将 PC12 细胞以每孔 5000 个细胞的密度接种在 384 孔板中,并置于含有 3 g/ml Tm 的 40μL 无酚红培养基中。通过机器人针转移,将 100 nL DiverSet E(5 mg/ml,溶于 DMSO)或结构多样性组和开放集合(10 mM,溶于 DMSO)(NCI) 添加到孔中。 48 小时后,使用基于发光的 ATP 测定来确定细胞的活力。在每个板上,用 DMSO 或 zVAD.fmk 处理的孔分别作为 ER 应激诱导 ATP 损失的阴性和阳性对照的救援。
1. eIF2α磷酸化检测(western blot):HeLa细胞(5×10⁵细胞/6孔板)经Salubrinal(0.1–10 μM)处理2小时,用含磷酸酶抑制剂的RIPA缓冲液裂解,30 μg蛋白经10% SDS-PAGE分离后转膜,用抗磷酸化eIF2α(Ser51)和总eIF2α抗体孵育,后续结合HRP标记二抗,通过光密度法定量条带强度,计算磷酸化eIF2α/总eIF2α比值 [1, 2] 2. 蛋白合成实验(³⁵S-蛋氨酸掺入):HeLa细胞(1×10⁵细胞/24孔板)经Salubrinal(0.1–10 μM)处理1小时,在无蛋氨酸DMEM中加入³⁵S-蛋氨酸(10 μCi/mL)孵育30分钟,裂解细胞后通过液体闪烁计数检测³⁵S掺入蛋白的量,蛋白合成速率以溶媒组为对照进行标准化 [1] 3. 心肌细胞活力实验(MTT):新生大鼠心室肌细胞(NRVM,1×10⁴细胞/96孔板)经Salubrinal(0.5–5 μM)预处理1小时,再经模拟I/R处理(缺氧2小时:1% O₂、5% CO₂、94% N₂;复氧2小时:21% O₂、5% CO₂)。加入MTT试剂(0.5 mg/mL)孵育4小时,DMSO溶解甲臜结晶后在570 nm处测吸光度,活力计算为(处理组吸光度/溶媒组吸光度)× 100% [2] 4. 神经元存活实验(NeuN免疫荧光):原代小鼠皮质神经元(5×10³细胞/盖玻片)经Salubrinal(0.5–5 μM)+红藻氨酸(100 μM)处理24小时,4%多聚甲醛固定后用抗NeuN抗体(神经元特异性标志物)和DAPI(核染色)染色,荧光显微镜下计数存活神经元(NeuN⁺/DAPI⁺),存活率以溶媒组为对照进行标准化 [3] |
| 动物实验 |
DMEM; 75μM; On cornea Eight-week-old male CD-1 outbred mice
1. Rat Myocardial I/R Model: Male Sprague-Dawley rats (250–300 g) were anesthetized with pentobarbital sodium (50 mg/kg, ip). The left anterior descending coronary artery (LAD) was ligated with a 6-0 silk suture for 30 minutes (ischemia), then the suture was loosened for 24 hours (reoxygenation). Salubrinal was dissolved in DMSO (10% v/v) + normal saline, and administered as a single intravenous injection (1 mg/kg) at the start of reoxygenation; vehicle group received DMSO/saline. After 24 hours, rats were euthanized, hearts were excised, and infarct size was measured by TTC staining (1% TTC in PBS, 37°C for 15 minutes) [2] 2. Mouse Excitotoxicity Model: Male ICR mice (20–25 g) were acclimated for 7 days. Salubrinal was dissolved in DMSO (5% v/v) + normal saline, and administered via intraperitoneal injection (5 mg/kg) 30 minutes before kainic acid (30 mg/kg, ip, dissolved in normal saline). Seizure severity was scored every 30 minutes for 4 hours using the Racine scale (1–5 grades). 24 hours after kainic acid injection, mice were euthanized, brains were fixed with 4% paraformaldehyde, embedded in paraffin, and sectioned (5 μm) for NeuN staining [3] 3. Mouse Chronic Toxicity Model: Female C57BL/6 mice (20–22 g) were divided into 2 groups (n=6/group): vehicle (DMSO/saline, ip) and Salubrinal (5 mg/kg, ip, daily for 7 days). Mice were weighed daily, and general behavior was observed. On day 8, mice were euthanized, and liver, kidney, and brain tissues were collected for western blot (CHOP, GRP78) and histopathological examination (H&E staining) [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
1. In vitro toxicity: Salubrinal (up to 20 μM) had no significant cytotoxicity in HeLa cells, MEFs, NRVMs, or primary cortical neurons (cell viability > 80% vs. vehicle, MTT/NeuN assay). No induction of excessive ER stress (e.g., CHOP overexpression) was observed at therapeutic concentrations (0.5–10 μM) [1, 2, 3]
2. In vivo acute toxicity: Rats (1 mg/kg, iv) and mice (5 mg/kg, ip) treated with Salubrinal showed no abnormal behavior (e.g., lethargy, ataxia), weight loss (<5% vs. baseline), or organ damage (liver, kidney, brain) within 24–72 hours. Serum biochemical markers (ALT, AST, BUN, creatinine) were within normal ranges [2, 3] 3. In vivo chronic toxicity: Mice treated with Salubrinal (5 mg/kg, ip, daily for 7 days) had no significant weight change, histopathological abnormalities in liver/kidney/brain, or upregulation of pro-apoptotic markers (CHOP), indicating low chronic toxicity [3] |
| 参考文献 | |
| 其他信息 |
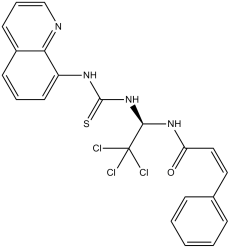
Salubrinal is a member of the class of quinolines that is a mixed aminal resulting from the formal condensation oftrichloroacetaldehyde with the amide nitrogen of trans-cinnamamide and the primary amino group of 1-quinolin-8-ylthiourea. It is a selective inhibitor of cellular complexes that dephosphorylate eukaryotic translation initiation factor 2 subunit alpha (eIF2alpha). It is a member of quinolines, a member of thioureas, an aminal, an organochlorine compound and a secondary carboxamide. It is functionally related to a trichloroacetaldehyde and a trans-cinnamamide.
1. Background: Salubrinal was identified via high-throughput screening in 2005 as the first small-molecule inhibitor of eIF2α dephosphorylation. It is a key research tool for studying the PERK-eIF2α branch of the unfolded protein response (UPR), a cellular pathway that mitigates endoplasmic reticulum (ER) stress [1] 2. Mechanism of action: Salubrinal specifically binds to GADD34-PP1c and CReP-PP1c complexes, blocking their ability to dephosphorylate phospho-eIF2α (eIF2α-P). Sustained eIF2α-P levels inhibit global protein synthesis (reducing ER protein load) while promoting translation of ATF4 (a transcription factor that induces expression of ER stress-protective genes, e.g., GRP78, HO-1), thereby enhancing cell tolerance to ER stress [1, 2] 3. Research applications: Salubrinal is widely used in preclinical models of ER stress-related diseases, including myocardial ischemia-reperfusion injury, neurodegenerative diseases (e.g., Alzheimer’s, Parkinson’s), diabetes, and sepsis. It has not entered clinical development and is primarily used as a research reagent [1, 2, 3] 4. Limitations: Salubrinal has low potency (IC₅₀ ~1.5 μM) and poor solubility, limiting its use in high-throughput studies. It cannot distinguish between GADD34-PP1c and CReP-PP1c, making it difficult to dissect the specific roles of these two complexes in UPR. No FDA-approved indications or safety warnings exist, as it is not a clinical drug [1, 3] |
| 分子式 |
C21H17CL3N4OS
|
|
|---|---|---|
| 分子量 |
479.81
|
|
| 精确质量 |
478.018
|
|
| 元素分析 |
C, 52.57; H, 3.57; Cl, 22.17; N, 11.68; O, 3.33; S, 6.68
|
|
| CAS号 |
405060-95-9
|
|
| 相关CAS号 |
|
|
| PubChem CID |
5717801
|
|
| 外观&性状 |
Light brown to gray solid powder
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 折射率 |
1.732
|
|
| LogP |
6.21
|
|
| tPSA |
98.14
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
30
|
|
| 分子复杂度/Complexity |
622
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
ClC(C([H])(N([H])C(/C(/[H])=C(\[H])/C1C([H])=C([H])C([H])=C([H])C=1[H])=O)N([H])C(N([H])C1=C([H])C([H])=C([H])C2C([H])=C([H])C([H])=NC1=2)=S)(Cl)Cl
|
|
| InChi Key |
LCOIAYJMPKXARU-VAWYXSNFSA-N
|
|
| InChi Code |
InChI=1S/C21H17Cl3N4OS/c22-21(23,24)19(27-17(29)12-11-14-6-2-1-3-7-14)28-20(30)26-16-10-4-8-15-9-5-13-25-18(15)16/h1-13,19H,(H,27,29)(H2,26,28,30)/b12-11+
|
|
| 化学名 |
(E)-3-phenyl-N-[2,2,2-trichloro-1-(quinolin-8-ylcarbamothioylamino)ethyl]prop-2-enamide
|
|
| 别名 |
Salubrinal
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 10 mg/mL (20.84 mM) in 45% PEG300 +5% Tween-80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0842 mL | 10.4208 mL | 20.8416 mL | |
| 5 mM | 0.4168 mL | 2.0842 mL | 4.1683 mL | |
| 10 mM | 0.2084 mL | 1.0421 mL | 2.0842 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|---|
|
|