| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
Sennoside A 能够抑制多种版本的 RDDP 和 RNase H。据观察,各种 RDDP 变体的 IC50 值分别为 78 μM (K103N RT)、21.3 μM (Y181C RT) 和 64 μM (Y188L RT)。 。 N474A RT 的 IC50 值分别为 18.4 μM,Q475A RT 的 IC50 值分别为 17.7 μM[3]。 HIV-1 重组 CAT 病毒采用实验室适应的 T 向性病毒 HXBc2 的包膜糖蛋白进行假型化,感染 Jurka 细胞。受感染细胞的 CAT 活性受到 sensnoside A(5–20 μM;72 小时)的极大抑制[3]。
|
||
|---|---|---|---|
| 体内研究 (In Vivo) |
Sensnoside A(25 mg/kg、50 mg/kg;灌胃 12 周)改变了 2 型糖尿病(T2D)小鼠的肠道微生物组组成,也具有抗肥胖作用[3]。在有遗传缺陷的动物的回肠中,Sensnoside A 还能提高紧密连接蛋白并减少炎症[3]。
|
||
| 动物实验 |
|
||
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Use of senna in the recommended doses for a limited period of time has been associated with few side effects, most of which are mild and transient and related to its laxative action. With longer term and higher dose use of senna, however, adverse events have been described including several cases of clinically apparent liver injury. The time to onset of liver injury was usually after 3 to 5 months of use, and the pattern of serum enzyme elevations was hepatocellular. The liver injury was usually mild-to-moderate in severity and resolved rapidly with discontinuation. In at least one instance, reexposure led to rapid recurrence of liver injury. Immunoallergic features and autoimmune markers were not present in the published cases. In addition, a related plant commonly known as coffee senna or Cassia orientalis has been linked to many instances of acute, severe toxicity with encephalopathy, myopathy and hepatic dysfunction. Outbreaks of “hepato-myo-encephopathy” of unknown cause among children occurred yearly in Uttar Pradesh, India typically between September and November. Investigation eventually identified Cassia occidentalis ingestion as the probable cause, typically occurring in children who eat the leaves or pods of the common flowering weed. While Cassia occidentalis has also been used to prepare tea, the amount ingested was minimal. In children, and rarely in adults, the presentation was precipitous with nausea, vomiting, tremor, abnormal and violent behavior, grimacing and self-mutilation followed by stupor and coma at which time serum aminotransferase and bilirubin levels were typically elevated. In severe instances, the liver injury was progressive, serum ammonia and INR levels rose and patients developed coma, convulsions and status epilepticus that was unresponsive to therapy. Autopsies revealed hepatic necrosis and cholestasis. A similar pattern of symptoms and injury occurs in animals that consume Cassia occidentalis. Whether this syndrome has a similar pathogenesis to the rare instance of hepatic injury attributed to typical senna (Cassia acutifolia or angustifolio) that is used as a laxative is unknown. Likelihood score: D (possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Although an early uncontrolled report using an old senna product found increased frequency of diarrhea in breastfed infants, several controlled studies using modern senna products found no effect on the infant. Usual doses of senna are acceptable to use during breastfeeding. ◉ Effects in Breastfed Infants After administration of 3.6 mL of senna fluidextract on day 5 postpartum, a laxative effect on the bowels was observed in 6 of 10 infants. In another observational study, no cases of diarrhea were observed among the breastfed infants of 148 mothers who received 2 teaspoonfuls of Senokot (equivalent to 700 mg of senna pod) on day 3 postpartum. Fifty mothers who were in the first day postpartum received senna equal to 450 mg of senna pod. Additional doses were given on subsequent days if needed. None of their breastfed infants were noted to have any markedly abnormal stools, although all of the infants also received supplemental feedings. In a randomized, nonblinded study, 35 mothers were given tablets containing a total of 14 mg of standardized senna extract once daily for 2 weeks starting in the immediate postpartum period. Six of the 37 breastfed infants were reported to have diarrhea which was a higher percentage than with other nonabsorbable laxatives in the study. Sixteen women were given 800 mg of powdered senna containing 24 mg of sennosides. None of their breastfed infants had any abnormal stools. A randomized, double-blind trial compared commercial senna tablets (Senokot) in a dose of 2 tablets (14 mg sennosides a and b) twice daily for 8 doses started on the first day postpartum to placebo. Of the women in the study, 126 breastfed their infants and took senna while 155 control mothers breastfed their infants. There was no difference in the percentages of infants in the active and control groups with loose stools or diarrhea. Twenty postpartum mothers were given a laxative containing plantango seeds (psyllium) and senna equivalent to 15 mg of sennosides a and b daily on days 2 to 4 postpartum. Of the 11 infants who were breastfed, none had any loose stools. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
||
| 参考文献 |
|
||
| 其他信息 |
Senna (powdered) is a yellow-brown powder with a slight odor and taste. (NTP, 1992)
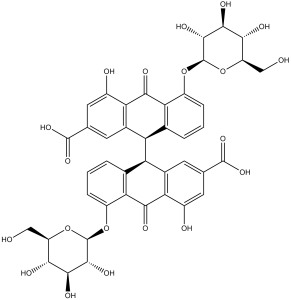
Sennoside A is a member of the class of sennosides that is rel-(9R,9'R)-9,9',10,10'-tetrahydro-9,9'-bianthracene-2,2'-dicarboxylic acid which is substituted by hydroxy groups at positions 4 and 4', by beta-D-glucopyranosyloxy groups at positions 5 and 5', and by oxo groups at positions 10 and 10'. The exact stereochemisty at positions 9 and 9' is not known - it may be R,R (as shown) or S,S. It is a member of sennosides and an oxo dicarboxylic acid. Senna (Cassia species) is a popular herbal laxative that is available without prescription. Senna is generally safe and well tolerated, but can cause adverse events including clinically apparent liver injury when used in high doses for longer than recommended periods. Sennoside A has been reported in Rheum palmatum, Rheum tanguticum, and other organisms with data available. Senokot is a standardized, concentrated preparation, by Purdue, containing the anthraquinone glycosides sennosides extracted from senna leaves with laxative activity. Sennosides act on and irritate the lining of the intestine wall, thereby causing increased intestinal muscle contractions leading to vigorous bowel movement. Preparations of SENNA PLANT. They contain sennosides, which are anthraquinone type CATHARTICS and are used in many different preparations as laxatives. |
| 分子式 |
C42H38O20
|
|
|---|---|---|
| 分子量 |
862.74
|
|
| 精确质量 |
862.195
|
|
| CAS号 |
81-27-6
|
|
| 相关CAS号 |
|
|
| PubChem CID |
73111
|
|
| 外观&性状 |
Light yellow to yellow solid powder
|
|
| 密度 |
1.7±0.1 g/cm3
|
|
| 沸点 |
1144.8±65.0 °C at 760 mmHg
|
|
| 熔点 |
200-240ºC
|
|
| 闪点 |
348.6±27.8 °C
|
|
| 蒸汽压 |
0.0±0.3 mmHg at 25°C
|
|
| 折射率 |
1.763
|
|
| LogP |
1.88
|
|
| tPSA |
347.96
|
|
| 氢键供体(HBD)数目 |
12
|
|
| 氢键受体(HBA)数目 |
20
|
|
| 可旋转键数目(RBC) |
9
|
|
| 重原子数目 |
62
|
|
| 分子复杂度/Complexity |
1550
|
|
| 定义原子立体中心数目 |
12
|
|
| SMILES |
C1=CC2=C(C(=C1)O[C@H]3[C@@H]([C@H]([C@@H]([C@H](O3)CO)O)O)O)C(=O)C4=C([C@@H]2[C@@H]5C6=C(C(=CC=C6)O[C@H]7[C@@H]([C@H]([C@@H]([C@H](O7)CO)O)O)O)C(=O)C8=C5C=C(C=C8O)C(=O)O)C=C(C=C4O)C(=O)O
|
|
| InChi Key |
IPQVTOJGNYVQEO-KGFNBKMBSA-N
|
|
| InChi Code |
InChI=1S/C42H38O20/c43-11-23-31(47)35(51)37(53)41(61-23)59-21-5-1-3-15-25(17-7-13(39(55)56)9-19(45)27(17)33(49)29(15)21)26-16-4-2-6-22(60-42-38(54)36(52)32(48)24(12-44)62-42)30(16)34(50)28-18(26)8-14(40(57)58)10-20(28)46/h1-10,23-26,31-32,35-38,41-48,51-54H,11-12H2,(H,55,56)(H,57,58)/t23-,24-,25-,26-,31-,32-,35+,36+,37-,38-,41-,42-/m1/s1
|
|
| 化学名 |
(9R)-9-[(9R)-2-carboxy-4-hydroxy-10-oxo-5-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-9H-anthracen-9-yl]-4-hydroxy-10-oxo-5-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-9H-anthracene-2-carboxylic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 6.25 mg/mL (7.24 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,将 100 μL 62.5 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (2.41 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.1591 mL | 5.7955 mL | 11.5910 mL | |
| 5 mM | 0.2318 mL | 1.1591 mL | 2.3182 mL | |
| 10 mM | 0.1159 mL | 0.5795 mL | 1.1591 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02665624 | Completed | Drug: Senna and stewed apricot juice Drug: Senna alone |
Colonoscopy Preparation | Camlıca Erdem Hospital | April 2015 | Phase 4 |
| NCT02239510 | Terminated Has Results | Drug: Senna Drug: Linzess |
Chronic Idiopathic Constipation | TriHealth Inc. | September 2014 | Not Applicable |
| NCT00571896 | Completed | Drug: Senna+ docusate Drug: placebo |
Constipation | Hartford Hospital | January 2008 | Phase 2 Phase 3 |
| NCT02008864 | Completed | Drug: Senna Drug: Placebo |
End Stage Renal Disease Pruritus |
Shiraz University of Medical Sciences | August 2011 | Not Applicable |