| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
HMG-CoA reductase (Ki = 0.2 nM); Simvastatin competitively inhibits HMG-CoA reductase with a Ki of 0.1–0.2 nM in cell-free assays [1]
|
|---|---|
| 体外研究 (In Vitro) |
辛伐他汀是一种无活性的药物前体,需要在肝脏中分解成羟基酸形式才能开始发挥作用。它本身没有药物活性。在体外测试中,氢氧化钠(NaOH)可以激活它。
辛伐他汀的体外激活[13,14] 方法1[13]:辛伐他汀(5mg)可以通过在乙醇/氢氧化钠溶液中溶解来激活,在预热至50°C的水浴中孵育2小时。用去离子水将药物稀释至1 mL,并用盐酸(0.1 M)将pH值调至7。 方法2[14]:辛伐他汀是一种非活性内酯产品,须在50°C以下溶解在0.1 N氢氧化钠/乙醇中(2小时),才能转化为活性β-羟基酸形式。随后用盐酸(0.1 M)将pH中和至 7.2。 Simvastatin 的 IC50 值分别为 19.3 nM、13.3 nM 和 15.6 nM,抑制小鼠 LM 细胞、大鼠 H4II E 细胞和人 Hep G2 细胞中胆固醇的合成[1]。 30分钟内,辛伐他汀以剂量依赖性方式增加Akt的丝氨酸473磷酸化;峰值磷酸化发生在 1.0 µM[2]。辛伐他汀 (1.0 μM) 抑制无血清培养基发生细胞凋亡,加速血管结构的形成,并增加内源性 Akt 底物内皮一氧化氮合酶 (eNOS) 的磷酸化[2]。辛伐他汀具有抗炎特性,可减少 10 μM 的 IFN-γ 释放,以及抗 CD3/抗 CD28 抗体诱导的 PB 衍生单核细胞和类风湿性关节炎血液滑膜氟细胞的增殖 [3]。此外,大约 30% 通过同源接触产生的细胞介导的巨噬细胞 TNF-γ 释放被辛伐他汀 (10 μM) 阻断[3]。在星形胶质细胞和神经母细胞瘤细胞中,辛伐他汀 (5 μM) 显着降低星形胶质细胞中 ABCA1 表达、载脂蛋白 E 表达,并增强 SK-N-SH 细胞中糖原合成酶激酶 3β 和细胞周期蛋白依赖性激酶 5 表达 [7]。辛伐他汀可以抑制外泌体的释放[10]。辛伐他汀在 32 和 64 μM 时可减缓肿瘤细胞发育并使其停止在 G0/G1 期; 24、48 和 72 小时[11]。在 HepG2 和 Huh7 细胞中,辛伐他汀(32 和 64 μM;48 小时)会导致细胞凋亡[11]。 - HMG-CoA还原酶抑制: 1. 细胞实验:辛伐他汀在小鼠L-M成纤维细胞(IC50 = 19.3 nM)、大鼠H4IIE肝细胞(IC50 = 13.3 nM)和人HepG2细胞(IC50 = 15.6 nM)中抑制胆固醇合成 [1] - Akt激活: 1. Western Blot分析:内皮细胞经辛伐他汀(1.0 μM)处理30分钟后,剂量依赖性诱导Akt在Ser473位点磷酸化,增强内皮型一氧化氮合酶(eNOS)的磷酸化 [2] - 炎症调节: 1. PBMC增殖实验:辛伐他汀(10 μM)减少抗CD3/CD28刺激的外周血单核细胞(PBMCs)和类风湿关节炎患者滑膜液细胞的增殖,抑制IFN-γ释放约30% [3] 2. 单核细胞迁移实验:辛伐他汀(10 μM)通过减少趋化因子CCL2和CXCL10的分泌,降低单核细胞跨人血脑屏障(BBB)内皮细胞的迁移 [9] - 阿尔茨海默病相关基因表达: 1. 人星形胶质细胞/神经元细胞:辛伐他汀显著降低ABCA1(星形胶质细胞中减少79%,神经母细胞瘤细胞中减少97%)和载脂蛋白E(ApoE)的表达,同时增加神经元细胞中tau蛋白的表达 [7] |
| 体内研究 (In Vivo) |
口服给药时,辛伐他汀可抑制放射性标记的乙酸盐转化为胆固醇,IC50 为 0.2 mg/kg[1]。在喂食富含致动脉粥样硬化胆固醇饮食的兔子中,辛伐他汀(4 毫克/天,口服 13 周)可将总胆固醇、低密度脂蛋白胆固醇和高密度脂蛋白胆固醇的增加逆转至正常水平[4]。在饲喂含有 0.25% 胆固醇的饮食的兔子中,辛伐他汀 (6 mg/kg) 会增加肝脏 LDL 受体的数量和 LDL 受体依赖性结合[5]。在饲喂致动脉粥样硬化饮食的食蟹猴中,辛伐他汀(20 mg/kg/天)导致病变处巨噬细胞含量减少 1.3 倍,血管细胞粘附分子-1、白细胞介素-1β 和组织因子表达减少 2 倍。这些减少伴随着病变平滑肌细胞和胶原蛋白含量增加 2.1 倍[6]。辛伐他汀治疗(口服灌胃;每日一次;14天); 15 和 30 mg/kg)可减少氧化损伤、TNF-a 和 IL-6 水平,并恢复线粒体酶复合物的活性[12]。
- 家兔高胆固醇血症: 1. 胆固醇喂养模型:口服辛伐他汀(4 mg/天,持续13周)使胆固醇喂养家兔的血清总胆固醇、LDL-C和HDL-C水平恢复正常,减少动脉粥样硬化病变形成 [4] 2. LDL受体上调:辛伐他汀(6 mg/kg)增加家兔肝脏LDL受体结合和表达,增强LDL清除 [5] - 炎症性关节炎: 1. 胶原诱导关节炎模型:辛伐他汀(10 mg/kg/天,口服)减轻大鼠关节肿胀和组织学炎症,降低促炎细胞因子(TNF-α、IL-1β)水平 [3] - 肝脏缺血再灌注损伤: 1. 大鼠模型:辛伐他汀预处理(20 mg/kg,腹腔注射)在热缺血前维持肝脏ATP水平,减少ALT/AST释放,通过KLF2依赖性上调eNOS、血栓调节蛋白(TM)和血红素加氧酶-1(HO-1)减轻氧化应激 [8] - 血脑屏障通透性: 1. 人BBB模型:辛伐他汀(9.5×10-8 M)在体外降低人BBB内皮细胞对牛血清白蛋白和[¹⁴C]-蔗糖的通透性,限制白细胞迁移 [9] |
| 酶活实验 |
- HMG-CoA还原酶活性测定 [1]:
1. 反应体系:重组HMG-CoA还原酶(10 nM)与辛伐他汀(0.1–10 μM)及放射性标记的[³H]-HMG-CoA(100 μM)在缓冲液(50 mM Tris-HCl, pH 7.5, 10 mM MgCl₂, 1 mM DTT)中孵育。 2. 产物检测:通过液体闪烁计数测量[³H]-HMG-CoA转化为甲羟戊酸的量,得出辛伐他汀的Ki为0.1–0.2 nM [1] 为了在体外评估Akt蛋白激酶活性,将底物(2μg组蛋白H2B或25μg eNOS肽)与使用山羊多克隆抗Akt1抗体从细胞裂解物中免疫沉淀的Akt一起孵育。在将辛伐他汀添加到最终浓度的ATP(50μM)(含有10μCi的32P-γATP、二硫treitol(1mM)、HEPES缓冲液(20mM,pH 7.4)、MnCl2(10mM)、MgCl2(10 mM))后,启动激酶反应。在30°C下孵育30分钟后,在SDS-PAGE(15%)和放射自显影后,磷酸化组蛋白H2B可见。为了估计32P掺入eNOS肽的程度,通过在磷酸纤维素圆盘过滤器上点样来测量每个反应混合物,并通过切伦科夫计数来测量掺入的磷酸盐的量。野生型肽序列是1174-RIRTQSFSLQERHLRGAVPWA-1194,并且突变体eNOS肽是相同的,除了丝氨酸1179被丙氨酸取代[3]。 |
| 细胞实验 |
细胞增殖测定[11]
细胞类型: HepG2 和 Huh7 细胞 测试浓度: 32 和 64 μM 孵育时间: 24、48 和 72 小时 实验结果:与对照相比,抑制肿瘤细胞生长(ctrl,p<0.05)。 细胞凋亡分析[11] 细胞类型: HepG2 和 Huh7 细胞 测试浓度: 32 和 64 μM 孵育持续时间:48 小时 实验结果:早期凋亡率从未处理的 ctrl 细胞中的 9.2% 增加到 18.2% (32 μM) 和 19.8% (64 μM) 分别将晚期细胞凋亡从 ctrl 细胞中的 35.0% 增加到 HepG2 细胞中的 56.9% (32 μM) 和 48.0% (64 μM)。 细胞周期分析[11] 细胞类型: HepG2 和 Huh7 细胞 测试浓度: 32 和 64 μM <孵化持续时间:24、48 和 72 小时 实验结果:与 ctrl 肿瘤相比,CDK1、CDK2、CDK4 以及细胞周期蛋白 D1 和 E 下调细胞。 - Akt磷酸化实验 [2]: 1. 细胞培养:人脐静脉内皮细胞(HUVECs)饥饿过夜后,用辛伐他汀(0.1–10 μM)处理30分钟。 2. Western Blot:使用特异性抗体检测磷酸化Akt(p-Akt)和总Akt,显示1.0 μM时p-Akt达到峰值 [2] - 血脑屏障通透性实验 [9]: 1. Transwell系统:人BBB内皮细胞在Transwell小室中培养,经辛伐他汀(10 μM)处理后,通过荧光法检测70 kDa FITC-葡聚糖的通透性,显示降低50–60% [9] |
| 动物实验 |
Animal/Disease Models: Male wistar rats with oxidative damage by Intrastriatal 6-OHDA administration[12]
Doses: 15 and 30 mg/kg Route of Administration: po (oral gavage); 15 and 30 mg/kg; one time/day; 14 days Experimental Results: Attenuated oxidative damage (decreased MDA, nitrite levels and restoration of decreased GSH), attenuated TNF-a and IL-6 levels, and restored itochondrial enzyme complex activities as compared to 6-OHDA group. - Rabbit Hypercholesterolemia Model [4]: 1. Diet and Treatment: Rabbits were fed a 1% cholesterol diet for 13 weeks. Simvastatin (4 mg/day) was administered orally via gastric gavage. 2. Sample Collection: Serum lipids were measured at baseline and weekly intervals; aortic lesions were analyzed histologically [4] - Rat Liver Ischemia-Reperfusion Model [8]: 1. Pretreatment: Rats received simvastatin (20 mg/kg, i.p.) 30 minutes before 30 minutes of hepatic ischemia. 2. Reperfusion: Livers were cold-stored in UW solution for 24 hours, then reperfused ex vivo for 60 minutes. Hepatic ATP, ALT/AST, and oxidative stress markers were assessed [8] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Peak plasma concentrations of both active and total inhibitors were attained within 1.3 to 2.4 hours post-dose. While the recommended therapeutic dose range is 10 to 40 mg/day, there was no substantial deviation from linearity of AUC with an increase in dose to as high as 120 mg. Relative to the fasting state, the plasma profile of inhibitors was not affected when simvastatin was administered immediately before a test meal. In a pharmacokinetic study of 17 healthy Chinese volunteers, the major PK parameters were as follows: Tmax 1.44 hours, Cmax 9.83 ug/L, t1/2 4.85 hours, and AUC 40.32ug·h/L. Simvastatin undergoes extensive first-pass extraction in the liver, the target organ for the inhibition of HMG-CoA reductase and the primary site of action. This tissue selectivity (and consequent low systemic exposure) of orally administered simvastatin has been shown to be far greater than that observed when the drug is administered as the enzymatically active form, i.e. as the open hydroxyacid. In animal studies, after oral dosing, simvastatin achieved substantially higher concentrations in the liver than in non-target tissues. However, because simvastatin undergoes extensive first-pass metabolism, the bioavailability of the drug in the systemic system is low. In a single-dose study in nine healthy subjects, it was estimated that less than 5% of an oral dose of simvastatin reached the general circulation in the form of active inhibitors. Genetic differences in the OATP1B1 (Organic-Anion-Transporting Polypeptide 1B1) hepatic transporter encoded by the SCLCO1B1 gene (Solute Carrier Organic Anion Transporter family member 1B1) have been shown to impact simvastatin pharmacokinetics. Evidence from pharmacogenetic studies of the c.521T>C single nucleotide polymorphism (SNP) showed that simvastatin plasma concentrations were increased on average 3.2-fold for individuals homozygous for 521CC compared to homozygous 521TT individuals. The 521CC genotype is also associated with a marked increase in the risk of developing myopathy, likely secondary to increased systemic exposure. Other statin drugs impacted by this polymorphism include [rosuvastatin], [pitavastatin], [atorvastatin], [lovastatin], and [pravastatin]. For patients known to have the above-mentioned c.521CC OATP1B1 genotype, a maximum daily dose of 20mg of simvastatin is recommended to avoid adverse effects from the increased exposure to the drug, such as muscle pain and risk of rhabdomyolysis. Evidence has also been obtained with other statins such as [rosuvastatin] that concurrent use of statins and inhibitors of Breast Cancer Resistance Protein (BCRP) such as elbasvir and grazoprevir increased the plasma concentration of these statins. Further evidence is needed, however a dose adjustment of simvastatin may be necessary. Other statin drugs impacted by this polymorphism include [fluvastatin] and [atorvastatin]. Following an oral dose of 14C-labeled simvastatin in man, 13% of the dose was excreted in urine and 60% in feces. Rat studies indicate that when radiolabeled simvastatin was administered, simvastatin-derived radioactivity crossed the blood-brain barrier. Both simvastatin and its beta-hydroxyacid metabolite are highly bound (approximately 95%) to human plasma proteins. Rat studies indicate that when radiolabeled simvastatin was administered, simvastatin-derived radioactivity crossed the blood-brain barrier. /MILK/ It is not known whether simvastatin is distributed into human breast milk ... . Following an oral dose of (14)C-labeled simvastatin in man, 13% of the dose was excreted in urine and 60% in feces. Plasma concentrations of total radioactivity (simvastatin plus (14)C-metabolites) peaked at 4 hours and declined rapidly to about 10% of peak by 12 hours postdose. Since simvastatin undergoes extensive first-pass extraction in the liver, the availability of the drug to the general circulation is low (<5%). Absorption, distribution and excretion of (14)C-simvastatin were studied in male rats after 21-day consecutive daily oral administration at the dose of 10 mg/kg. Plasma levels of (14C)simvastatin at 1hr after each administration did not increase during and after repeated administration. The radioactivity levels-time curve after the final administration was similar to that after the first dosing. The cumulative excretion of radioactivity in urine and feces accounted for 9.0% and 91.4% of the total dose, respectively, within 96hr after the final administration. After the final administration, radioactivity was concentrated in the gastrointestinal tracts, liver and kidney. The distribution pattern was similar to that observed after the single administration. There was no accumulation of the drug and its metabolites in the tissues of rats after the consecutive oral administration of (14)C-simvastatin. Foeto-placental transfer and excretion of radioactivity into milk were studied in pregnant and lactating rats after single oral administration of (14)C-simvastatin. Whole body autoradiograms of rats on day 12 and 18 of gestation showed low distribution and rapid elimination of radioactivity from the fetus. On day 18 of gestation, the concentration of radioactivity in the placenta, amniotic fluid and fetal tissues were nearly equal to or less than those in the maternal plasma. The amount of radioactivity transferred into a fetus was about 0.02% of the oral dose. The concentrations of radioactivity in the milk were about 20-54% of those in maternal plasma. For more Absorption, Distribution and Excretion (Complete) data for Simvastatin (6 total), please visit the HSDB record page. Metabolism / Metabolites Simvastatin is administered as the inactive lactone derivative that is then metabolically activated to its β-hydroxyacid form by a combination of spontaneous chemical conversion and enzyme-mediated hydrolysis by nonspecific carboxyesterases in the intestinal wall, liver, and plasma. Oxidative metabolism in the liver is primarily mediated by CYP3A4 and CYP3A5, with the remaining metabolism occurring through CYP2C8 and CYP2C9. The major active metabolites of simvastatin are β-hydroxyacid metabolite and its 6'-hydroxy, 6'-hydroxymethyl, and 6'-exomethylene derivatives. Polymorphisms in the CYP3A5 gene have been shown to affect the disposition of simvastatin and may provide a plausible explanation for interindividual variability of simvastatin disposition and pharmacokinetics. The major active metabolites of simvastatin present in human plasma are the beta-hydroxyacid of simvastatin and its 6'-hydroxy, 6'-hydroxymethyl, and 6'-exomethylene derivatives. Simvastatin has known human metabolites that include 6'-alpha-Hydroxysimvastatin, 6'-exomethylene, and 3', 5'-Dihydrodiol. Hepatic, simvastatin is a substrate for CYP3A4. The major active metabolites of simvastatin are ‘_-hydroxyacid metabolite and its 6'-hydroxy, 6'-hydroxymethyl, and 6'-exomethylene derivatives Route of Elimination: Following an oral dose of 14C-labeled simvastatin in man, 13% of the dose was excreted in urine and 60% in feces. Half Life: 3 hours Biological Half-Life 4.85 hours - Absorption: 1. Oral Bioavailability: Simvastatin has low oral bioavailability (~5%) due to extensive first-pass hepatic metabolism [1] - Metabolism: 1. Cytochrome P450: Primarily metabolized by CYP3A4 to active metabolites (e.g., simvastatin acid), which contribute to HMG-CoA reductase inhibition [5] - Half-Life: 1. Plasma Half-Life: ~2 hours for parent compound; active metabolites have a half-life of ~19 hours [1] - Excretion: 1. Biliary Elimination: ~60% of the dose is excreted in feces; ~13% in urine [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Simvastatin is anticholesteremic agent and Hydroxymethylglutaryl-CoA reductase Inhibitor. HUMAN EXPOSURE AND TOXICITY: Simvastatin occasionally causes myopathy manifested as muscle pain, tenderness or weakness with creatine kinase above ten times the upper limit of normal. Myopathy sometimes takes the form of rhabdomyolysis with or without acute renal failure secondary to myoglobinuria, and rare fatalities have occurred. The risk of myopathy is increased by high levels of statin activity in plasma. Predisposing factors for myopathy include advanced age (>/=65 years), female gender, uncontrolled hypothyroidism, and renal impairment. Although myopathy, including rhabdomyolysis, is a known adverse effect of all statins, studies have shown that patients receiving higher dosages of simvastatin may be at greater risk of muscle injury than those receiving lower dosages of the drug and possibly other statins. ANIMAL STUDIES: Significant lethality was observed in mice after a single oral dose of 9 g/sq m. No evidence of lethality was observed rats or dogs treated with doses of 30 and 100 g/sq m, respectively. No specific diagnostic signs were observed in rodents. At these doses the only signs seen in dogs were emesis and mucoid stools. Optic nerve degeneration was seen in clinically normal dogs treated with simvastatin for 14 weeks at 180 mg/kg/day, a dose that produced mean plasma drug levels about 12 times higher than the mean plasma drug level in humans taking 80 mg/day. There were cataracts in female rats after two years of treatment with 50 and 100 mg/kg/day and in dogs after three months at 90 mg/kg/day and at two years at 50 mg/kg/day. An increased incidence of thyroid follicular adenomas was observed in female rats receiving simvastatin for 2 years. In mice receiving simvastatin dosages of 25, 100, and 400 mg/kg daily for 72 weeks, there was an increased incidence of liver carcinomas in females receiving 400 mg/kg daily and in males receiving 100 and 400 mg/kd daily. The maximum incidence of liver carcinomas was 90% in male mice. An increased incidence of liver adenomas was observed in female mice receiving 100 and 400 mg/kg daily. The incidence of lung adenomas was increased in mice receiving 100 and 400 mg/kg daily, regardless of gender, and the incidence of adenomas of the Harderian gland (a gland of the rodent eye) was increased in mice receiving 400 mg/kd daily. A tumorigenic effect was not observed in mice receiving 25 mg/kg daily in this study. CNS vascular lesions, characterized by perivascular hemorrhage and edema, mononuclear cell infiltration of perivascular spaces, perivascular fibrin deposits and necrosis of small vessels were seen in dogs treated with simvastatin at a dose of 360 mg/kg/day. Decreased fertility was observed in male rats receiving simvastatin 25 mg/kg daily for 34 weeks. This effect was not observed in a subsequent study using the same dosage for 11 weeks (the entire duration of the spermatogenesis cycle in rats, including epididymal maturation). No microscopic changes in the testes were observed in either study. At a simvastatin dosage of 180 mg/kg daily in rats, seminiferous tubule degeneration was observed. Simvastatin did not exhibit mutagenic potential in vitro in microbial mutagen (Ames) tests using mutant strains of Salmonella typhimurium with or without rat or mouse liver metabolic activation, the alkaline elution assay using rat hepatocytes, a V-79 mammalian cell forward mutation study, a chromosome aberration study in Chinese hamster ovary cells, or in vivo in a chromosomal aberration assay in mouse bone marrow. Simvastatin is a prodrug in which the 6-membered lactone ring of simvastatin is hydrolyzed in vivo to generate the beta,delta-dihydroxy acid, an active metabolite structurally similar to HMG-CoA (hydroxymethylglutaryl CoA). Once hydrolyzed, simvastatin competes with HMG-CoA for HMG-CoA reductase, a hepatic microsomal enzyme. Interference with the activity of this enzyme reduces the quantity of mevalonic acid, a precursor of cholesterol. Hepatotoxicity Up to 5% of patients taking simvastatin chronically may experience minor elevations in serum ALT levels during therapy, but confirmed elevations to above three times the upper limit of normal (ULN) occur in only 1% to 2% of patients. These abnormalities are usually asymptomatic and self-limited even if therapy is continued. ALT elevations are clearly more frequent in patients taking higher doses of simvastatin (40 and 80 mg daily). In several studies, ALT elevations were no more frequent in patients taking 10 and 20 mg of simvastatin daily than in placebo recipients. Clinically apparent liver injury due to simvastatin is rare. The usual latency to onset of symptoms of liver disease ranges from one week to as long as 3 years, but most cases have a latency of 1 to 6 months. The pattern of injury is variable, hepatocellular, cholestatic or mixed patterns have been described. Immunoallergic symptoms of fever and rash are uncommon. Isolated cases of an autoimmune hepatitis-like syndrome associated with simvastatin therapy have been reported, some of which did not reverse completely with discontinuation, resulting in a chronic hepatitis requiring long term immunosuppressive therapy. In most cases, however, recovery occurs within 1 to 3 months. Rare cases of acute liver failure and death have been attributed to simvastatin. But in view of the wide use of simvastatin, clinically apparent liver injury is exceeding rare and is estimated to occur in 1 per 100,000 patient years of exposure. Likelihood score: A (well known but rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No relevant published information exists on the use of simvastatin during breastfeeding. Because of a concern with disruption of infant lipid metabolism, the consensus is that simvastatin should not be used during breastfeeding. However, others have argued that children homozygous for familial hypercholesterolemia are treated with statins beginning at 1 year of age, that statins have low oral bioavailability, and risks to the breastfed infant are low, especially with rosuvastatin and pravastatin.[1] Until more data become available, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Both simvastatin and its β-hydroxyacid metabolite are highly bound (approximately 95%) to human plasma proteins. Interactions Statins are widely prescribed to organ transplant recipients with hyperlipidemia. We report the case of a cardiac transplant recipient who developed severe rhabdomyolysis and acute renal failure after being switched from pravastatin to simvastatin. The patient's other medications included cyclosporin A and diltiazem. Unlike pravastatin, the metabolism and tissue concentrations of simvastatin-and of other statins - can be greatly affected by these drugs. ... Strong CYP3A4 inhibitors: Simvastatin, like several other inhibitors of HMG-CoA reductase, is a substrate of CYP3A4. Simvastatin is metabolized by CYP3A4 but has no CYP3A4 inhibitory activity; therefore it is not expected to affect the plasma concentrations of other drugs metabolized by CYP3A4. Elevated plasma levels of HMG-CoA reductase inhibitory activity increases the risk of myopathy and rhabdomyolysis, particularly with higher doses of simvastatin. Concomitant use of drugs labeled as having a strong inhibitory effect on CYP3A4 is contraindicated. If treatment with itraconazole, ketoconazole, posaconazole, voriconazole, erythromycin, clarithromycin or telithromycin is unavoidable, therapy with simvastatin must be suspended during the course of treatment. In one study, concomitant administration of digoxin with simvastatin resulted in a slight elevation in digoxin concentrations in plasma. Patients taking digoxin should be monitored appropriately when simvastatin is initiated The risk of myopathy, including rhabdomyolysis, is increased by concomitant administration of amiodarone, dronedarone, ranolazine, or calcium channel blockers such as verapamil, diltiazem, or amlodipine. For more Interactions (Complete) data for Simvastatin (19 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat (female) sc 672 mg/kg LD50 Rat (male) sc 1088 mg/kg LD50 Rat (male) ip 898 mg/kg LD50 Rat (female) ip 705 mg/kg For more Non-Human Toxicity Values (Complete) data for Simvastatin (13 total), please visit the HSDB record page. - Hepatotoxicity: 1. Rat Studies: Chronic administration of simvastatin (20–40 mg/kg/day for 30 days) increased serum ALT/AST and hepatic lipid peroxidation, reversed by co-administration with naringenin [7] - Myopathy Risk: 1. Drug Interactions: Concurrent use with CYP3A4 inhibitors (e.g., cyclosporine) increases plasma simvastatin levels, elevating risk of rhabdomyolysis [5] - Carcinogenicity: 1. Mouse Studies: High-dose simvastatin (400 mg/kg/day) increased hepatic adenoma/carcinoma incidence in mice, likely due to peroxisome proliferator-activated receptor (PPAR) activation [1] |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Anticholesteremic Agents; Hydroxymethylglutaryl-CoA Reductase Inhibitors /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Simvastatin is included in the database. Zocor is indicated as an adjunct to diet to reduce total cholesterol (total-C), low-density lipoprotein cholesterol (LDL-C), and Apo B levels in adolescent boys and girls who are at least one year post-menarche, 10-17 years of age, with HeFH, if after an adequate trial of diet therapy the following findings are present: LDL cholesterol remains >/= 190 mg/dL; or LDL cholesterol remains >/= 160 mg/dL and there is a positive family history of premature cardiovascular disease (CVD) or two or more other CVD risk factors are present in the adolescent patient. The minimum goal of treatment in pediatric and adolescent patients is to achieve a mean LDL-C <130 mg/dL. The optimal age at which to initiate lipid-lowering therapy to decrease the risk of symptomatic adulthood CAD has not been determined. /Included in US product label/ Zocor is indicated to: Reduce elevated total cholesterol (total-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (Apo B), and triglycerides (TG), and to increase high-density lipoprotein cholesterol (HDL-C) in patients with primary hyperlipidemia (Fredrickson type IIa, heterozygous familial and nonfamilial) or mixed dyslipidemia (Fredrickson type IIb); Reduce elevated TG in patients with hypertriglyceridemia (Fredrickson type lV hyperlipidemia); Reduce elevated TG and VLDL-C in patients with primary dysbetalipoproteinemia (Fredrickson type III hyperlipidemia); Reduce total-C and LDL-C in patients with homozygous familial hypercholesterolemia (HoFH) as an adjunct to other lipid-lowering treatments (e.g., LDL apheresis) or if such treatments are unavailable. For more Therapeutic Uses (Complete) data for Simvastatin (11 total), please visit the HSDB record page. Drug Warnings Zocor is contraindicated in women who are or may become pregnant. Lipid lowering drugs offer no benefit during pregnancy, because cholesterol and cholesterol derivatives are needed for normal fetal development. Atherosclerosis is a chronic process, and discontinuation of lipid-lowering drugs during pregnancy should have little impact on long-term outcomes of primary hypercholesterolemia therapy. ... Serum cholesterol and triglycerides increase during normal pregnancy, and cholesterol or cholesterol derivatives are essential for fetal development. Because statins decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, Zocor may cause fetal harm when administered to a pregnant woman. If Zocor is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Grapefruit juice contains one or more components that inhibit CYP3A4 and can increase the plasma levels of drugs metabolized by CYP3A4. The effect of typical consumption (one 250-ml glass daily) is minimal (13% increase in active plasma HMG-CoA reductase inhibitory activity as measured by the area under the concentration-time curve) and of no clinical relevance. However, because larger quantities significantly increase the plasma levels of HMG-CoA reductase inhibitory activity, grapefruit juice should be avoided during simvastatin therapy. It is not known whether simvastatin is excreted in human milk. Because a small amount of another drug in this class is excreted in human milk and because of the potential for serious adverse reactions in nursing infants, women taking simvastatin should not nurse their infants. A decision should be made whether to discontinue nursing or discontinue drug, taking into account the importance of the drug to the mother. Because advanced age (>/= 65 years) is a predisposing factor for myopathy, including rhabdomyolysis, Zocor should be prescribed with caution in the elderly. In a clinical trial of patients treated with simvastatin 80 mg/day, patients >/= 65 years of age had an increased risk of myopathy, including rhabdomyolysis, compared to patients <65 years of age. For more Drug Warnings (Complete) data for Simvastatin (33 total), please visit the HSDB record page. Pharmacodynamics Simvastatin is an oral antilipemic agent which inhibits HMG-CoA reductase. It is used to lower total cholesterol, low density lipoprotein-cholesterol (LDL-C), apolipoprotein B (apoB), non-high density lipoprotein-cholesterol (non-HDL-C), and trigleride (TG) plasma concentrations while increasing HDL-C concentrations. High LDL-C, low HDL-C and high TG concentrations in the plasma are associated with increased risk of atherosclerosis and cardiovascular disease. The total cholesterol to HDL-C ratio is a strong predictor of coronary artery disease and high ratios are associated with higher risk of disease. Increased levels of HDL-C are associated with lower cardiovascular risk. By decreasing LDL-C and TG and increasing HDL-C, rosuvastatin reduces the risk of cardiovascular morbidity and mortality. Elevated cholesterol levels, and in particular, elevated low-density lipoprotein (LDL) levels, are an important risk factor for the development of CVD. Use of statins to target and reduce LDL levels has been shown in a number of landmark studies to significantly reduce the risk of development of CVD and all-cause mortality. Statins are considered a cost-effective treatment option for CVD due to their evidence of reducing all-cause mortality including fatal and non-fatal CVD as well as the need for surgical revascularization or angioplasty following a heart attack. Evidence has shown that even for low-risk individuals (with <10% risk of a major vascular event occurring within 5 years) statins cause a 20%-22% relative reduction in major cardiovascular events (heart attack, stroke, coronary revascularization, and coronary death) for every 1 mmol/L reduction in LDL without any significant side effects or risks. **Skeletal Muscle Effects** Simvastatin occasionally causes myopathy manifested as muscle pain, tenderness or weakness with creatine kinase (CK) above ten times the upper limit of normal (ULN). Myopathy sometimes takes the form of rhabdomyolysis with or without acute renal failure secondary to myoglobinuria, and rare fatalities have occurred. Predisposing factors for myopathy include advanced age (≥65 years), female gender, uncontrolled hypothyroidism, and renal impairment. Chinese patients may also be at increased risk for myopathy. In most cases, muscle symptoms and CK increases resolved when treatment was promptly discontinued. In a clinical trial database of 41,413 patients, the incidence of myopathy was approximately 0.03% and 0.08% at 20 and 40 mg/day, respectively, while the risk of myopathy with simvastatin 80 mg (0.61%) was disproportionately higher than that observed at the lower doses. It's therefore recommended that the 80mg dose of simvastatin should be used only in patients who have been taking simvastatin 80 mg chronically (e.g., for 12 months or more) without evidence of muscle toxicity. As well, patients already stabilized on simvastatin 80mg should be monitored closely for evidence of muscle toxicity; if they need to be initiated on an interacting drug that is contraindicated or is associated with a dose cap for simvastatin, that patient should be switched to an alternative statin with less potential for the drug-drug interaction. The risk of myopathy during treatment with simvastatin may be increased with concurrent administration of interacting drugs such as [fenofibrate], [niacin], [gemfibrozil], [cyclosporine], and strong inhibitors of the CYP3A4 enzyme. Cases of myopathy, including rhabdomyolysis, have been reported with HMG-CoA reductase inhibitors coadministered with [colchicine], and caution should therefore be exercised when prescribing these two medications together. **Liver Enzyme Abnormalities** Persistent increases (to more than 3X the ULN) in serum transaminases have occurred in approximately 1% of patients who received simvastatin in clinical studies. When drug treatment was interrupted or discontinued in these patients, the transaminase levels usually fell slowly to pretreatment levels. The increases were not associated with jaundice or other clinical signs or symptoms. In the Scandinavian Simvastatin Survival Study (4S), the number of patients with more than one transaminase elevation to >3 times the ULN, over the course of the study, was not significantly different between the simvastatin and placebo groups (14 [0.7%] vs. 12 [0.6%]). The frequency of single elevations of ALT to 3 times the ULN was significantly higher in the simvastatin group in the first year of the study (20 vs. 8, p=0.023), but not thereafter. In the HPS (Heart Protection Study), in which 20,536 patients were randomized to receive simvastatin 40 mg/day or placebo, the incidences of elevated transaminases (>3X ULN confirmed by repeat test) were 0.21% (n=21) for patients treated with simvastatin and 0.09% (n=9) for patients treated with placebo. **Endocrine Effects** Increases in HbA1c and fasting serum glucose levels have been reported with HMG-CoA reductase inhibitors, including simvastatin. Although cholesterol is the precursor of all steroid hormones, studies with simvastatin have suggested that this agent has no clinical effect on steroidogenesis. Simvastatin caused no increase in biliary lithogenicity and, therefore, would not be expected to increase the incidence of gallstones. - Mechanism of Action: 1. Dual Effects: Simvastatin inhibits cholesterol biosynthesis via HMG-CoA reductase and promotes angiogenesis/vasodilation through Akt/eNOS activation [2] 2. KLF2-Dependent Protection: In liver ischemia-reperfusion injury, simvastatin upregulates KLF2, which transcriptionally activates anti-inflammatory and cytoprotective genes (e.g., eNOS, HO-1) [8] - Indications: 1. Primary Use: Treatment of hypercholesterolemia to reduce LDL-C and cardiovascular risk [1] 2. Off-Label Uses: Neuroprotection in stroke, anti-inflammatory therapy in rheumatoid arthritis, and attenuation of BBB permeability in multiple sclerosis [3,9] - FDA Warnings: 1. Muscle Toxicity: Risk of myopathy/rhabdomyolysis, particularly with high doses or co-administration of CYP3A4 inhibitors [1] |
| 分子式 |
C25H38O5
|
|---|---|
| 分子量 |
418.57
|
| 精确质量 |
418.271
|
| 元素分析 |
C, 71.74; H, 9.15; O, 19.11
|
| CAS号 |
79902-63-9
|
| 相关CAS号 |
Simvastatin-d6;1002347-71-8;Simvastatin-d11;1002347-74-1;Simvastatin-d3;1002347-61-6; 139893-43-9 (ammonium); 79902-63-9 (free); 101314-97-0 (sodium)
|
| PubChem CID |
54454
|
| 外观&性状 |
White to off-white crystalline powder from n-butyl chloride + hexane
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
564.9±50.0 °C at 760 mmHg
|
| 熔点 |
139 °C
|
| 闪点 |
184.8±23.6 °C
|
| 蒸汽压 |
0.0±3.5 mmHg at 25°C
|
| 折射率 |
1.530
|
| LogP |
4.41
|
| tPSA |
72.83
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
706
|
| 定义原子立体中心数目 |
7
|
| SMILES |
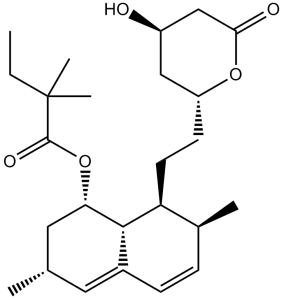
O([C@H]1C[C@@H](C)C=C2C=C[C@@H]([C@@H]([C@@H]12)CC[C@H]1OC(=O)C[C@H](O)C1)C)C(=O)C(C)(C)CC
|
| InChi Key |
RYMZZMVNJRMUDD-OVOOIQHOSA-N
|
| InChi Code |
InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23?/m0/s1
|
| 化学名 |
(1S,3R,7S,8S)-8-(2-((2R,4R)-4-hydroxy-6-oxotetrahydro-2H-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate
|
| 别名 |
MK-0733, MK 0733, MK0733, Zocor; Synvinolin; MK 733; Sinvacor; MK-733; MK733; Simvastatin; Denan; Lipex;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.97 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (5.97 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.97 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.5 mg/mL (5.97 mM) (饱和度未知) in 10% EtOH + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清乙醇储备液加入到 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: ≥ 2.5 mg/mL (5.97 mM) (饱和度未知) in 10% EtOH + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 EtOH 储备液加入900 μL 玉米油中,混合均匀。 配方 6 中的溶解度: 2% DMSO+30% PEG 300+5% Tween80+ddH2O:10 mg/mL 配方 7 中的溶解度: 10 mg/mL (23.89 mM) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3891 mL | 11.9454 mL | 23.8909 mL | |
| 5 mM | 0.4778 mL | 2.3891 mL | 4.7782 mL | |
| 10 mM | 0.2389 mL | 1.1945 mL | 2.3891 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05542095 | Withdrawn | Drug: Simvastatin | Olfactory Disorder COVID-19 |
Washington University School of Medicine |
May 1, 2023 | Phase 1 |
| NCT06178640 | Not yet recruiting | Drug: Simvastatin 40 mg film-coated tablet |
Healthy Volunteer | International Bio service | August 13, 2024 | Phase 1 |
| NCT05771675 | Not yet recruiting | Drug: Simvastatin Drug: Placebo |
Recurrent Acute Pancreatitis | Cedars-Sinai Medical Center | January 2024 | Early Phase 1 |
| NCT05550415 | Recruiting | Drug: Simvastatin 40mg Drug: Placebo |
Chemotherapy Effect Simvastatin Adverse Reaction |
Indonesia University | August 19, 2022 | Phase 2 |
|
|---|
|
|
|
|---|
|
|