| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
PDGFRβ (IC50 = 2 nM); VEGFR2 (IC50 = 80 nM); FLT3; c-Kit
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:Sunitinib 还有效抑制 Kit 和 FLT-3。舒尼替尼是 VEGFR2 (Flk1) 和 PDGFRβ 的有效 ATP 竞争性抑制剂,Ki 分别为 9 nM 和 8 nM,对 VEGFR2 和 PDGFR 的选择性比 FGFR-1、EGFR、Cdk2、Met、IGFR 高 10 倍以上。 1、Abl 和 src。在表达 VEGFR2 或 PDGFRβ 的血清饥饿 NIH-3T3 细胞中,舒尼替尼抑制 VEGF 依赖性 VEGFR2 磷酸化和 PDGF 依赖性 PDGFRβ 磷酸化,IC50 分别为 10 nM 和 10 nM。 Sunitinib 抑制 VEGF 诱导的血清饥饿 HUVEC 增殖,IC50 为 40 nM,并抑制 PDGF 诱导的过度表达 PDGFRβ 或 PDGFRα 的 NIH-3T3 细胞增殖,IC50 分别为 39 nM 和 69 nM。 Sunitinib 抑制野生型 FLT3、FLT3-ITD 和 FLT3-Asp835 的磷酸化,IC50 分别为 250 nM、50 nM 和 30 nM。 Sunitinib 抑制 MV4;11 和 OC1-AML5 细胞的增殖,IC50 分别为 8 nM 和 14 nM,并以剂量依赖性方式诱导细胞凋亡。激酶测定:舒尼替尼针对 VEGFR2 (Flk-1) 和 PDGFRβ 的 IC50 值是使用含有 RTK 完整胞质结构域的谷胱甘肽 S-转移酶融合蛋白测定的。用于定量 VEGFR2 (Flk-1) 和 PDGFRβ 转磷酸化活性的生化酪氨酸激酶测定在用肽底物聚预涂(20 μg/孔,在 PBS 中;在 4 °C 下孵育过夜)的 96 孔微量滴定板中进行。谷氨酸、酪氨酸 (4:1)。添加 1-5% (w/v) BSA 的 PBS 溶液可封闭多余的蛋白质结合位点。纯化的 GST 融合蛋白在杆状病毒感染的昆虫细胞中产生。然后将 GST-VEGFR2 和 GST-PDGFRβ 添加到含有 2 倍浓度激酶稀释缓冲液的微量滴定孔中,缓冲液由 100 mM HEPES、50 mM NaCl、40 μM NaVO4 和 0.02% (w/v) BSA 组成。 GST-VEGFR2 或 GST-PDGFRβ 的最终酶浓度为 50 ng/mL。随后将 25 μL 稀释的舒尼替尼添加到每个反应孔中,以产生适合每种酶的一系列抑制剂浓度。通过在 MnCl2 溶液中添加不同浓度的 ATP 来启动激酶反应,使得最终 ATP 浓度跨越酶的 Km,并且 MnCl2 的最终浓度为 10 mM。将板在室温下孵育 5-15 分钟,然后添加 EDTA 终止反应。然后用TBST洗涤板3次。将兔多克隆抗磷酸酪氨酸抗血清按 1:10,000 稀释在含有 0.5% (w/v) BSA、0.025% (w/v) 脱脂奶粉和 100 μM NaVO4 的 TBST 中添加到孔中,并在 37° 下孵育 1 小时C。然后用TBST洗涤板3次,然后添加与辣根过氧化物酶缀合的山羊抗兔抗血清(在TBST中1:10,000稀释)。将板在 37°C 下孵育 1 小时,然后用 TBST 洗涤 3 次。添加 2,2'-连氮基-二-[3-乙基苯并噻唑啉磺酸]作为底物后,对每孔中磷酸酪氨酸的量进行定量。细胞测定:在添加舒尼替尼和 FL(50 ng/mL;仅限 FLT3-WT 细胞)之前,将细胞在含有 0.1% FBS 的培养基中饥饿过夜。培养 48 小时后,使用 Alamar Blue 测定或台盼蓝细胞活力测定来测量增殖。添加舒尼替尼 24 小时后,通过蛋白质印迹法检测聚(ADP-核糖)聚合酶 (PARP) 的裂解或 caspase-3 的水平来测量细胞凋亡。
|
| 体内研究 (In Vivo) |
与体内对 VEGFR2 或 PDGFR 磷酸化和信号传导的实质性和选择性抑制一致,舒尼替尼(20-80 mg/kg/天)对包括 HT-29 在内的多种肿瘤异种移植模型表现出广泛且有效的剂量依赖性抗肿瘤活性、A431、Colo205、H-460、SF763T、C6、A375 或 MDA-MB-435。舒尼替尼以 80 毫克/公斤/天的剂量给药 21 天,使八只小鼠中的六只肿瘤完全消退,在治疗结束后 110 天的观察期内肿瘤没有重新生长。舒尼替尼的第二轮治疗对于第一轮治疗期间未完全消退的肿瘤仍然有效。舒尼替尼治疗可显着降低肿瘤 MVD,SF763T 神经胶质瘤肿瘤减少约 40%。 SU11248 治疗可完全抑制表达荧光素酶的 PC-3M 异种移植物的额外肿瘤生长,尽管肿瘤大小没有减小。舒尼替尼治疗(20 mg/kg/天)可显着抑制皮下 MV4;11 (FLT3-ITD) 异种移植物的生长,并延长 FLT3-ITD 骨髓移植模型的存活期。
|
| 酶活实验 |
舒尼替尼针对 PDGFRβ 和 VEGFR2 (Flk-1) 的 IC50 值是通过使用包含整个 RTK 胞质结构域的谷胱甘肽 S-转移酶融合蛋白来确定的。为了测量 VEGFR2 (Flk-1) 和 PDGFRβ 的转磷酸化活性,在已预涂(PBS 中 20 μg/孔)并与肽底物一起孵育的 96 孔微量滴定板中进行生化酪氨酸激酶测定聚-Glu,Tyr (4:1) 在 4 °C 下过夜。在 PBS 中添加 1-5% (w/v) BSA 可阻断多余的蛋白质结合位点。感染杆状病毒的昆虫细胞产生纯化的 GST 融合蛋白。然后在微量滴定孔中填充 2 倍浓度激酶稀释缓冲液中的 GST-VEGFR2 和 GST-PDGFRβ,该缓冲液含有 40 μM NaVO4、50 mM NaCl、100 mM HEPES 和 0.02%(w/ v) BSA。 50 ng/mL 是 GST-VEGFR2 或 GST-PDGFRβ 的最终酶浓度。为了创建适合每种酶的抑制剂浓度范围,将 25 μL 稀释的舒尼替尼添加到每个反应孔中。将 MnCl2 溶液与不同浓度的 ATP 混合以启动激酶反应。 MnCl2 的最终浓度为 10 mM,最终 ATP 浓度跨越酶的 Km。让板在室温下静置五到十五分钟后,通过添加 EDTA 停止反应。然后用TBST洗板3次。将兔多克隆抗磷酸酪氨酸抗血清以 1:10,000 稀释度添加到含有 0.025% (w/v) 脱脂奶粉、0.5% (w/v) BSA 和 100 μM NaVO4 的 TBST 孔中后,将孔在 37°C 下孵育一小时。 TBST 洗涤 3 次后,用与辣根过氧化物酶缀合的山羊抗兔抗血清(1:10,000 稀释于 TBST)接种平板。 37°C 孵育一小时后,用 TBST 清洗板 3 次。添加 2,2'-连氮基-二-[3-乙基苯并噻唑啉磺酸]作为底物后,即可对每孔中磷酸酪氨酸的量进行定量。
|
| 细胞实验 |
在添加 FL(50 ng/mL;仅 FLT3-WT 细胞)和舒尼替尼之前,将细胞在含有 0.1% FBS 的培养基中饥饿过夜。培养 48 小时后,使用台盼蓝细胞活力测定或 Alamar Blue 测定评估增殖。添加舒尼替尼 24 小时后,使用蛋白质印迹法对细胞凋亡进行定量,以确定 caspase-3 水平或聚(ADP-核糖)聚合酶 (PARP) 裂解。
|
| 动物实验 |
Mice: The mice used are female nu/nu (8–12 weeks old, 25 grams). In short, on day 0, mice receive a subcutaneous injection of 3-5×106 tumor cells into the hind flank region. After tumors reach the indicated average size, mice bearing tumors are treated daily orally with carboxymethyl cellulose suspension or as a citrate buffered (pH 3.5) solution containing sunitinib. Tumor growth is assessed using tumor volume measurements taken twice a week. When tumors in animals receiving vehicle treatment reach an average size of 1000 mm3 or are determined to negatively impact the animals' quality of life, studies are usually stopped.
Rats: The Wistar rats are adult males weighing between 325 and 349 g. In two drug studies, the efficacy of the time-lapse imaging method in assessing the anti-angiogenic effects of a particular drug treatment is verified. First, mesenteric windows are taken from adult male Wistar rats, and the tissues are cultured for three days in two different experimental groups: 1) 10% serum (n = 8 tissues from 4 rats), and 2) 10% serum + Sunitinib (5 μM; n=8 tissues from 4 rats). |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Maximum plasma concentrations (Cmax) of sunitinib are generally observed between 6 and 12 hours (Tmax) following oral administration. Food has no effect on the bioavailability of sunitinib. Sunitinib may be taken with or without food. The pharmacokinetics were similar in healthy volunteers and in the solid tumor patient populations tested, including patients with GIST and RCC. Sunitinib is metabolized primarily by the cytochrome P450 enzyme, CYP3A4, to produce its primary active metabolite, which is further metabolized by CYP3A4. Elimination is primarily via feces. In a human mass balance study of [14C]sunitinib, 61% of the dose was eliminated in feces, with renal elimination accounting for 16% of the administered dose. 2230 L (apparent volume of distribution, Vd/F) 34 - 62 L/h [Total oral clearance] Following oral administration, peak plasma concentrations of sunitinib generally occur within 6-12 hours. Food has no effect on bioavailability of sunitinib. Steady-state concentrations of sunitinib and its primary active metabolite are achieved within 10 to 14 days. By Day 14, combined plasma concentrations of sunitinib and its active metabolite ranged from 62.9 - 101 ng/mL. No significant changes in the pharmacokinetics of sunitinib or the primary active metabolite were observed with repeated daily administration or with repeated cycles in the dosing regimens tested. Sunitinib and its primary active metabolite are 95 and 90% bound to human plasma proteins in vitro, respectively. The apparent volume of distribution (Vd/F) for sunitinib was 2230 L. In the dosing range of 25 - 100 mg, the area under the plasma concentration-time curve (AUC) and Cmax increase proportionately with dose. For more Absorption, Distribution and Excretion (Complete) data for Sunitinib (11 total), please visit the HSDB record page. Metabolism / Metabolites Sunitinib is metabolized primarily by the cytochrome P450 enzyme, CYP3A4, to produce its primary active metabolite, which is further metabolized by CYP3A4. Sunitinib is metabolized principally by cytochrome P-450 (CYP) isoenzyme 3A4 to several metabolites. The main circulating metabolite, an N-desethyl derivative, has been shown to be equipotent to sunitinib in biochemical and cellular assays; this metabolite accounts for approximately 23-37% of total plasma concentrations of the drug and also is metabolized by CYP3A4. Sunitinib and its primary active metabolite were the major drug-related compounds identified in plasma, urine, and feces, representing 91.5%, 86.4% and 73.8% of radioactivity in pooled samples, respectively. Biological Half-Life Following administration of a single oral dose in healthy volunteers, the terminal half-lives of sunitinib and its primary active metabolite are approximately 40 to 60 hours and 80 to 110 hours, respectively. Following oral administration of a single dose in healthy volunteers, the terminal half-life of sunitinib or its primary active metabolite is approximately 40-60 or 80-110 hours, respectively. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In large clinical trials of sunitinib, elevations in serum aminotransferase levels were common, occurring in 39% of sunitinib vs 23% of placebo recipients. Values greater than 5 times the upper limit of normal (ULN) occurred in only 2% to 3% of sunitinib recipients (and 1% of controls). These abnormalities were usually asymptomatic. Dose adjustment or temporary discontinuation and restarting at a lower dose is recommended if enzyme levels are markedly elevated (ALT or AST persistently greater than 5 times ULN or bilirubin more than 3 times ULN). Sunitinib therapy is also associated with a high rate of serum bilirubin elevations, generally in the mild-to-moderate range and not in association with ALT or AST elevations. These changes are probably due to interaction with hepatic UDP-glucuronyltransferase, the enzyme that is also responsible for bilirubin excretion. More importantly, there have been several case reports of clinically apparent liver injury attributed to sunitinib therapy. The time to onset was after several cycles of therapy. The pattern of serum enzyme elevations was typically hepatocellular and the clinical presentation resembled acute hepatic necrosis. In some instances, the injury may have been due to hypotension, shock or ischemia rather than direct hepatotoxicity (Case 1). Regardless, the injury can be severe and several instances of acute liver failure and death have been reported. Immunoallergic features (rash, fever and eosinophilia) are not common. Finally, sunitinib has also been reported to cause hyperammonemia and encephalopathy in rare patients with cancer treated with conventional or even low oral doses (Case 2). The time to onset was within 1 to 3 weeks, presenting with confusion and irritability with minimal elevations in serum enzymes and bilirubin and marked increases (4-10 times the ULN) in serum ammonia. Recovery is rapid once sunitinib is stopped and the syndrome can recur with re-exposure. Interesting, there appears to be little cross-reactivity to this complication with other tyrosine kinase inhibitors. Likelihood score: B (highly likely cause of clinically apparent liver injury, including hyperammonemic syndrome). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of sunitinib during breastfeeding. Because sunitinib and its metabolite are over 90% bound to plasma proteins, the amount in milk is likely to be low. However, one of its metabolites has a half-life of up to 110 hours, and might accumulate in the infant. The manufacturer recommends that breastfeeding be discontinued during sunitinib therapy and for at least 4 weeks after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Binding of sunitinib and its primary metabolite to human plasma protein in vitro was 95% and 90%, respectively. Interactions ... A 57-year-old woman who started sunitinib treatment for relapsed metastatic gastrointestinal stromal tumor after imatinib failure had disease stabilization and normal liver function through 8 cycles of sunitinib 50 mg/day for 4 weeks, followed by 2 weeks off treatment. Her continuing medications included acetaminophen approximately 4.5 g/wk, as well as standard medications for asthma. In cycle 8, she received oral levothyroxine 50-150 microg/day for approximately 30 days to control hypothyroidism before beginning cycle 9 of sunitinib. On day 4 of cycle 9, she was hospitalized with progressively rising circulating liver enzyme levels. She died 4 days postadmission despite discontinuation of sunitinib and initiation of intensive supportive treatment. At autopsy, her liver showed severe centrilobular necrosis with moderate-to-severe steatosis and minimal parenchymal invasion by the neoplasm. Viral stains were negative. Hepatic failure has been reported rarely in patients receiving sunitinib. Autopsy results excluded neoplastic disease progression and viral infection in the etiology of the event, and the patient may have died of the combined interaction of sunitinib, acetaminophen, and levothyroxine. Although sunitinib was not more than a possible hepatotoxin (Roussel Uclaf Causality Assessment Method) and may even have been hepatoprotective over a 48-week period against chronic intake of acetaminophen (probable hepatotoxin) by producing regional hypothyroidism within the liver, it is hypothesized that correction of the putative hepatic hypothyroidism with oral levothyroxine (possible hepatotoxin) and reinitiation of sunitinib treatment may have triggered hepatic necrosis. ... Strong CYP3A4 inhibitors such as ketoconazole may increase sunitinib plasma concentrations. Selection of an alternate concomitant medication with no or minimal enzyme inhibition potential is recommended. Concurrent administration of SUTENT with the strong CYP3A4 inhibitor, ketoconazole, resulted in 49% and 51% increases in the combined (sunitinib + primary active metabolite) Cmax and AUC0-infinity values, respectively, after a single dose of Sunitinib in healthy volunteers. Co-administration of sunitinib with strong inhibitors of the CYP3A4 family (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, voriconazole) may increase sunitinib concentrations. Grapefruit may also increase plasma concentrations of sunitinib. CYP3A4 inducers such as rifampin may decrease sunitinib plasma concentrations. Selection of an alternate concomitant medication with no or minimal enzyme induction potential is recommended. Concurrent administration of sunitinib with the strong CYP3A4 inducer, rifampin, resulted in a 23% and 46% reduction in the combined (sunitinib + primary active metabolite) Cmax and AUC0-infinity values, respectively, after a single dose of sunitinib in healthy volunteers. Co-administration of sunitinib with inducers of the CYP3A4 family (e.g., dexamethasone, phenytoin, carbamazepine, rifampin, rifabutin, rifapentin, phenobarbital) may decrease sunitinib concentrations. St. John's wort may cause unpredictable decreases in plasma sunitinib concentrations and should be avoided during sunitinib therapy. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Angiogenesis Inhibitors; Antineoplastic Agents Sunitinib malate is indicated for the treatment of gastrointestinal stromal tumor after disease progression on or intolerance to imatinib mesylate. /Included in US product label/ Sunitinib malate is indicated for the treatment of advanced renal cell carcinoma. /Included in US product label/ Sunitinib malate is indicated for the treatment of progressive, well-differentiated pancreatic neuroendocrine tumors in patients with unresectable locally advanced or metastatic disease. /Included in US product label/ Drug Warnings /BOXED WARNING/ HEPATOTOXICITY-Hepatotoxicity has been observed in clinical trials and post-marketing experience. This hepatotoxicity may be severe, and deaths have been reported. Sunitinib has been associated with hepatotoxicity, which may result in liver failure or death. Liver failure has been observed in clinical trials (7/2281 [0.3%]) and post-marketing experience. Liver failure signs include jaundice, elevated transaminases and/or hyperbilirubinemia in conjunction with encephalopathy, coagulopathy, and/or renal failure. Monitor liver function tests (ALT, AST, bilirubin) before initiation of treatment, during each cycle of treatment, and as clinically indicated. Sunitinib should be interrupted for Grade 3 or 4 drug-related hepatic adverse events and discontinued if there is no resolution. Do not restart Sunitinib if patients subsequently experience severe changes in liver function tests or have other signs and symptoms of liver failure. Safety in patients with ALT or AST >2.5 x ULN or, if due to liver metastases, >5.0 x ULN has not been established. Cardiovascular events, including heart failure, myocardial disorders and cardiomyopathy, some of which were fatal, have been reported through post-marketing experience. Among patients receiving sunitinib for metastatic renal cell cancer in the randomized trial, 21% had a left ventricular ejection fraction (LVEF) below the lower limit of normal, and 4% experienced a decline in LVEF (to a value below 50% or as a reduction greater than 20% from the baseline value). Left ventricular dysfunction was reported in 1% and congestive heart failure in less than 1% of patients receiving sunitinib. For more Drug Warnings (Complete) data for Sunitinib (33 total), please visit the HSDB record page. Pharmacodynamics Sunitinib is an oral, small-molecule, multi-targeted receptor tyrosine kinase (RTK) inhibitor that was approved by the FDA on January 26, 2006. |
| 分子式 |
C22H27FN4O2
|
|---|---|
| 分子量 |
398.47
|
| 精确质量 |
398.211
|
| 元素分析 |
C, 66.31; H, 6.83; F, 4.77; N, 14.06; O, 8.03
|
| CAS号 |
557795-19-4
|
| 相关CAS号 |
Sunitinib Malate;341031-54-7;Sunitinib-d10;1126721-82-1;Sunitinib-d4;1126721-79-6; 342641-94-5; 1275588-72-1 (mesylate) ; 1126641-10-8; 1327155-72-5 (HCl); 1221149-36-5 (acetate); 1332306-95-2 (oxalate)
|
| PubChem CID |
5329102
|
| 外观&性状 |
Yellow solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
572.1±50.0 °C at 760 mmHg
|
| 熔点 |
189-191ºC
|
| 闪点 |
299.8±30.1 °C
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
| 折射率 |
1.611
|
| LogP |
3.15
|
| tPSA |
77.23
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
29
|
| 分子复杂度/Complexity |
636
|
| 定义原子立体中心数目 |
0
|
| SMILES |
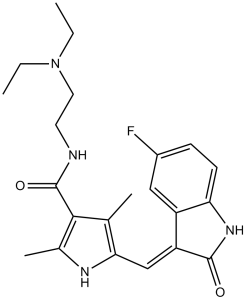
FC1C=C2C(NC(=O)/C/2=C\C2NC(C)=C(C(NCCN(CC)CC)=O)C=2C)=CC=1
|
| InChi Key |
WINHZLLDWRZWRT-ATVHPVEESA-N
|
| InChi Code |
InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12-
|
| 化学名 |
N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide
|
| 别名 |
SU11248; SU 11248; sunitinibum; Su-011248; Sunitinib Base; SU011248; SU-11248; sunitinib; trade name: Sutent.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.11 mg/mL (2.79 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 11.1 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1.11 mg/mL (2.79 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 11.1 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 5% DMSO+corn oil: 7mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5096 mL | 12.5480 mL | 25.0960 mL | |
| 5 mM | 0.5019 mL | 2.5096 mL | 5.0192 mL | |
| 10 mM | 0.2510 mL | 1.2548 mL | 2.5096 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
TAPUR: Testing the Use of Food and Drug Administration (FDA) Approved Drugs That Target a Specific Abnormality in a Tumor Gene in People With Advanced Stage Cancer
CTID: NCT02693535
Phase: Phase 2 Status: Recruiting
Date: 2024-11-12
|
 |