| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|

| 靶点 |
Bacterial protein synthesis; FGFR1 (IC50 = 9.3 nM); FGFR2 (IC50 = 7.6 nM); FGFR3 (IC50 = 22 nM); FGFR4 (IC50 = 290 nM)
|
|---|---|
| 体外研究 (In Vitro) |
替加环素(0.63-30 μM,预洗脱4天,处理72小时)抑制AML2细胞和HL-60细胞,IC50值分别为4.72±0.54和3.06±0.85 μM(新鲜生产)。偏置前一天,替加环素抑制 HL-60 细胞和 AML2 细胞,IC50 值为 4.27±0.45 μM 和 5.64±0.55 μM。替加环素抑制 AML2,IC50 为 5.02±0.60 和 4.39±0.44 μM(预偏置两天)。细胞和 HL-60 细胞的 IC50 值分别为 4.09±0.41 和 3.95±0.39 μM(预稀释三天)。在盐水中预稀释 4 天后,通过 CellTiter 面粉测量,替加环素抑制 TEX 人类细胞漂白的能力下降(从新鲜合成时的 IC50~5 μM)降至 IC50>50 μM [1]。
|
| 体内研究 (In Vivo) |
在 NOD/SCID 小鼠中,替加环素(50 mg/kg;腹腔注射;每天两次;持续 11 天)可减少肿瘤体积和重量 [1]。在生理盐水中,替加环素的血药峰浓度(Cmax)、终末半衰期(t1/2)、血药浓度-时间曲线下面积(AUC)、清除率(CL)和分布浓度(Vz)为,分别为22.8μg/mL、108.9min、1912.2min*μg/mL、26.1mL/min/kg、4109.4mL/kg。在替加环素制剂(60 mg/mL丙酮酸盐、3 mg/mL抗坏血酸、生理盐水)中,血浆峰浓度(Cmax)、血浆浓度-时间曲线下面积(AUC)、清除率(CL)和分布波形( Vz)为15.7μg/mL、110.3min、2036.5min*μg/mL、24.6mL/min/kg和3906.2mL/kg。
|
| 细胞实验 |
细胞活力测定[1]
细胞类型:人类白血病 OCI-AML2、HL-60 (ATCC) 和 TEX 细胞系 测试浓度: 0.63- 30 µM 孵育持续时间:预孵育 4 天,处理 72 小时 实验结果:抑制 AML2 细胞和HL-60细胞,IC50分别为4.72±0.54和3.06±0.85μM(新鲜制备)。 |
| 动物实验 |
Animal/Disease Models: NOD/SCID Mouse OCI-AML2 acute myeloid leukemia (AML) xenograft model [1]
Doses: 50 mg/kg Route of Administration: intraperitoneal (ip) injection; for 7) [1]. twice (two times) daily; continued for 11 days Experimental Results: Reduction in tumor volume and weight. Animal/Disease Models: NOD/SCID (severe combined immunodeficient) mouse[1] Doses: 50 mg/kg Route of Administration: intraperitoneal (ip) injection; 360 minutes Experimental Results: peak plasma concentration (Cmax), terminal half-life (t1/2), plasma concentration-time The area under the curve (AUC), clearance (CL) and distribution volume (Vz) were all 22.8 μg/108.9 minutes, 1912.2 minutes*μg/ml, 26.1 ml/minute/kg, and 4109.4 ml/kg respectively. |
| 参考文献 |
|
| 其他信息 |
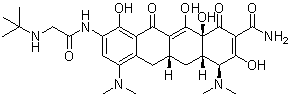
Tigecycline is tetracycline in which the hydroxy group at position 5 and the methyl group at position 6 are replaced by hydrogen, and with a dimethylamino substituent and an (N-tert-butylglycyl)amino substituent at positions 7 and 9, respectively. A glycylcycline antibiotic, it has activity against a broad range of Gram-positive and Gram-negative bacteria, including tetracycline-resistant organisms. It is used for the intravenous treatment of complicated skin and skin structure infections caused by susceptible organisms. It has a role as an antibacterial drug. It is a member of tetracyclines and a tertiary alpha-hydroxy ketone. It is a conjugate base of a tigecycline(1+).
Tigecycline is a Tetracycline-class Antibacterial. A tetracycline derivative that acts as a protein synthesis inhibitor. It is used as an antibacterial agent for the systemic treatment of complicated skin and intra-abdominal infections. It is also used for the treatment of community-acquired pneumonia. See also: Tigecycline (annotation moved to). |
| 分子式 |
C29H39N5O8
|
|---|---|
| 分子量 |
585.65
|
| 精确质量 |
585.279
|
| 元素分析 |
C, 59.47; H, 6.71; N, 11.96; O, 21.86
|
| CAS号 |
220620-09-7
|
| 相关CAS号 |
Tigecycline tetramesylate;Tigecycline hydrochloride;197654-04-9;Tigecycline mesylate;1135871-27-0;Tigecycline hydrate;1229002-07-6;Tigecycline-d9;2699607-86-6
|
| PubChem CID |
54686904
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
890.9±65.0 °C at 760 mmHg
|
| 熔点 |
164-166°C
|
| 闪点 |
492.6±34.3 °C
|
| 蒸汽压 |
0.0±0.3 mmHg at 25°C
|
| 折射率 |
1.675
|
| LogP |
-1.3
|
| tPSA |
205.76
|
| 氢键供体(HBD)数目 |
7
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
42
|
| 分子复杂度/Complexity |
1240
|
| 定义原子立体中心数目 |
4
|
| SMILES |
CC(C)(C)NCC(=O)NC1=CC(=C2C[C@H]3C[C@H]4[C@@H](C(=O)C(=C([C@]4(C(=O)C3=C(C2=C1O)O)O)O)C(=O)N)N(C)C)N(C)C
|
| InChi Key |
SOVUOXKZCCAWOJ-HJYUBDRYSA-N
|
| InChi Code |
InChI=1S/C29H39N5O8/c1-28(2,3)31-11-17(35)32-15-10-16(33(4)5)13-8-12-9-14-21(34(6)7)24(38)20(27(30)41)26(40)29(14,42)25(39)18(12)23(37)19(13)22(15)36/h10,12,14,21,31,36-37,40,42H,8-9,11H2,1-7H3,(H2,30,41)(H,32,35)/t12-,14-,21-,29-/m0/s1
|
| 化学名 |
(4S,4aS,5aR,12aR)-9-[[2-(tert-butylamino)acetyl]amino]-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4H-tetracene-2-carboxamide
|
| 别名 |
GAR-936; GAR936; Tigecycline; 220620-09-7; Tygacil; WAY-GAR-936; GAR-936; TYGACL; Tigecycline
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~25 mg/mL (~42.69 mM)
H2O : ~8.33 mg/mL (~14.22 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.27 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (3.55 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: 36.67 mg/mL (62.61 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7075 mL | 8.5375 mL | 17.0750 mL | |
| 5 mM | 0.3415 mL | 1.7075 mL | 3.4150 mL | |
| 10 mM | 0.1708 mL | 0.8538 mL | 1.7075 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Antibiotic Therapy for Infectious Diseases
CTID: NCT04937894
Phase: Status: Recruiting
Date: 2021-06-24
|
|
|