| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |

| 靶点 |
Bacterial protein synthesis; FGFR1 (IC50 = 9.3 nM); FGFR2 (IC50 = 7.6 nM); FGFR3 (IC50 = 22 nM); FGFR4 (IC50 = 290 nM)
|
|---|---|
| 体外研究 (In Vitro) |
替加环素(0.63-30 µM,预孵育 4 天后给药 72 小时)抑制 HL-60 细胞和 AML2 细胞,新生成的 IC50 值分别为 3.06±0.85 μM 和 4.72±0.54 μM。预孵育一天后,替加环素抑制 HL-60 细胞和 AML2 细胞,IC50 值分别为 4.27±0.45 μM 和 5.64±0.55 μM。预孵育两天后,替加环素抑制 AML2 细胞和 HL-60 细胞,IC50 值分别为 5.02±0.60 和 4.39±0.44 μM。预孵育三天后,替加环素抑制 HL-60 细胞和 AML2 细胞,IC50 值分别为 3.95±0.39 μM 和 4.09±0.41 μM。 CellTiter Flour 测试显示,在盐水中预孵育 4 天后,替加环素杀伤 TEX 人白血病细胞的能力下降(从新鲜制备时的 IC50~5 µM 到预孵育 4 天后的 IC50> 50 µM)[1]。
|
| 体内研究 (In Vivo) |
在 NOD/SCID 小鼠中,替加环素(50 mg/kg;腹腔注射;每天两次;持续 11 天)可减少肿瘤重量和体积 [1]。结果显示,置换时间为108.9分钟,血药峰浓度(Cmax)、终末半衰期(t1/2)、血药浓度-时间曲线下面积(AUC)、清除率(CL)、分布容积(Vz) 均为 22.8 μg/mL。在盐水中,加环素浓度依次为1912.2min*μg/mL、26.1mL/min/kg和4109.4mL/kg。制备过程中得到以下数值:血药峰浓度(Cmax)为15.7 μg/mL,终末半衰期t1/2,血药浓度-时间曲线下面积AUC,清除率CL,以及Vz 为分布体积。替加环素的浓度为 2036.5 min*μg/mL、24.6 mL/min/kg 和 3906.2 mL/kg(60 mg/mL 丙酮酸盐、3 mg/mL 抗坏血酸,pH 7,盐水中)[1]。
|
| 细胞实验 |
细胞活力测定[1]
细胞类型:人类白血病 OCI-AML2、HL-60 (ATCC) 和 TEX 细胞系 测试浓度: 0.63- 30 µM 孵育持续时间:预孵育 4 天,处理 72 小时 实验结果:抑制 AML2 细胞和HL-60细胞,IC50为4.72±0.54和3.06±0.85 μM(新鲜制备)。 |
| 动物实验 |
Animal/Disease Models: NOD/SCID mouse OCI-AML2 acute myeloid leukemia (AML) xenograft model [1]
Doses: 50 mg/kg Route of Administration: intraperitoneal (ip) injection; twice (two times) daily; for 11 days Experimental Results: Tumor volume and weight decrease. Animal/Disease Models: NOD/SCID (severe combined immunodeficient) mouse[1] Doses: 50 mg/kg Route of Administration: intraperitoneal (ip) injection; 360 minutes Experimental Results: peak plasma concentration (Cmax), terminal half-life (t1/2), plasma concentration-time The area under the curve (AUC), clearance (CL) and distribution volume (Vz) were all 22.8 μg/108.9 minutes, 1912.2 minutes*μg/ml, 26.1 ml/minute/kg, and 4109.4 ml/kg respectively. |
| 参考文献 |
|
| 其他信息 |
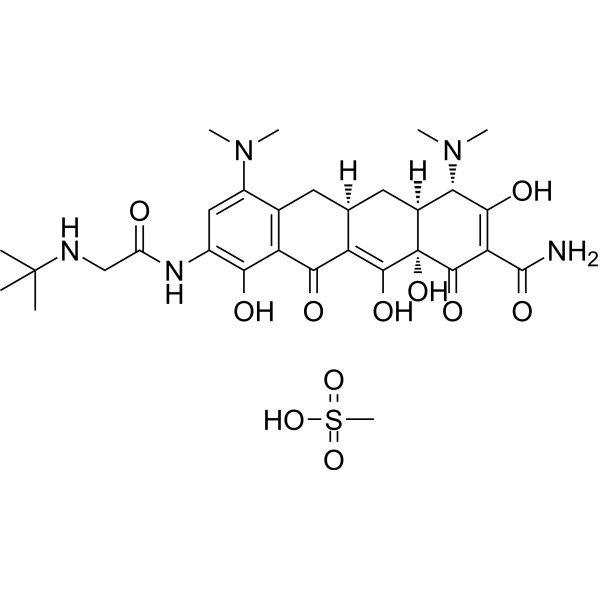
Tigecycline is tetracycline in which the hydroxy group at position 5 and the methyl group at position 6 are replaced by hydrogen, and with a dimethylamino substituent and an (N-tert-butylglycyl)amino substituent at positions 7 and 9, respectively. A glycylcycline antibiotic, it has activity against a broad range of Gram-positive and Gram-negative bacteria, including tetracycline-resistant organisms. It is used for the intravenous treatment of complicated skin and skin structure infections caused by susceptible organisms. It has a role as an antibacterial drug. It is a member of tetracyclines and a tertiary alpha-hydroxy ketone. It is a conjugate base of a tigecycline(1+).
Tigecycline is a Tetracycline-class Antibacterial. A tetracycline derivative that acts as a protein synthesis inhibitor. It is used as an antibacterial agent for the systemic treatment of complicated skin and intra-abdominal infections. It is also used for the treatment of community-acquired pneumonia. Tigecycline is a broad-spectrum, first-in-class glycylcycline antibiotic currently used to treat complicated skin and intra-abdominal infections, as well as community-acquired pneumonia. In addition, we have demonstrated that tigecycline also has in vitro and in vivo activity against acute myeloid leukemia (AML) due to its ability to inhibit mitochondrial translation. Tigecycline is relatively unstable after reconstitution, and this instability may limit the use of the drug in ambulatory infusions for the treatment of infection and may prevent the development of optimal dosing schedules for the treatment of AML. This study sought to identify a formulation that improved the stability of the drug after reconstitution and maintained its antimicrobial and antileukemic activity. A panel of chemical additives was tested to identify excipients that enhanced the stability of tigecycline in solution at room temperature for up to one week. We identified a novel formulation containing the oxygen-reducing agents ascorbic acid (3 mg/mL) and pyruvate (60 mg/mL), in saline solution, pH 7.0, in which tigecycline (1 mg/mL) remained intact when protected from light for at least 7 days. This formulation also preserved the drug's antibacterial and antileukemic activity in vitro. Moreover, the novel formulation retained tigecycline's antileukemic activity in vivo. Thus, we identified and characterized a novel formulation for tigecycline that preserves its stability and efficacy after reconstitution.[1] |
| 分子式 |
C30H43N5O11S
|
|---|---|
| 分子量 |
681.7543
|
| 精确质量 |
681.268
|
| CAS号 |
1135871-27-0
|
| 相关CAS号 |
Tigecycline;220620-09-7;Tigecycline tetramesylate;Tigecycline hydrochloride;197654-04-9;Tigecycline hydrate;1229002-07-6
|
| PubChem CID |
78358330
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| tPSA |
269
|
| 氢键供体(HBD)数目 |
8
|
| 氢键受体(HBA)数目 |
14
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
47
|
| 分子复杂度/Complexity |
1330
|
| 定义原子立体中心数目 |
4
|
| SMILES |
S(C([H])([H])[H])(=O)(=O)O[H].O([H])[C@@]12C(=C(C(N([H])[H])=O)C([C@]([H])([C@]1([H])C([H])([H])[C@]1([H])C([H])([H])C3=C(C([H])=C(C(=C3C(=C1C2=O)O[H])O[H])N([H])C(C([H])([H])N([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])=O)N(C([H])([H])[H])C([H])([H])[H])N(C([H])([H])[H])C([H])([H])[H])=O)O[H]
|
| InChi Key |
QRMALYUMKBOEGR-KXLOKULZSA-N
|
| InChi Code |
InChI=1S/C29H39N5O8.CH4O3S/c1-28(2,3)31-11-17(35)32-15-10-16(33(4)5)13-8-12-9-14-21(34(6)7)24(38)20(27(30)41)26(40)29(14,42)25(39)18(12)23(37)19(13)22(15)36;1-5(2,3)4/h10,12,14,21,31,36-37,40,42H,8-9,11H2,1-7H3,(H2,30,41)(H,32,35);1H3,(H,2,3,4)/t12-,14-,21-,29-;/m0./s1
|
| 化学名 |
(4S,4aS,5aR,12aR)-9-[[2-(tert-butylamino)acetyl]amino]-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4H-tetracene-2-carboxamide;methanesulfonic acid
|
| 别名 |
Tigecycline mesylate; 1135871-27-0; Tigecycline (mesylate); (4S,4aS,5aR,12aR)-9-[[2-(tert-butylamino)acetyl]amino]-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4H-tetracene-2-carboxamide;methanesulfonic acid; GAR-936 mesylate;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4668 mL | 7.3341 mL | 14.6681 mL | |
| 5 mM | 0.2934 mL | 1.4668 mL | 2.9336 mL | |
| 10 mM | 0.1467 mL | 0.7334 mL | 1.4668 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01789905 | COMPLETEDWITH RESULTS | Drug: Tigecycline (Tygacil) | Intra-Abdominal Infections Skin Disease, Infectious |
Pfizer | 2013-04-15 | |
| NCT02191475 | UNKNOWN STATUS | Drug: glycopeptide plus carbapenem Drug: Haizheng Li Xing ® plus tazocin ® |
Abdominal Infection | Tianjin Medical University Cancer Institute and Hospital |
2014-05 | Phase 2 Phase 3 |
| NCT00488488 | COMPLETEDWITH RESULTS | Drug: tigecycline | Infection | Pfizer | 2006-11 | |
| NCT02931526 | UNKNOWN STATUS | Drug: Tigecycline | Bacterial Infection Critically Ill |
Zhujiang Hospital | 2016-08 | |
| NCT02931526 | UNKNOWN STATUS | Drug: Tigecycline | Bacterial Infection Critically Ill |
Zhujiang Hospital | 2016-08 |