| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| 100g |
|
||
| 200g | |||
| Other Sizes |
| 靶点 |
Secondary bile acid metabolite; Endogenous Metabolite
|
|---|---|
| 体外研究 (In Vitro) |
通过 FXR 介导的 ACE2 调节,Ursodeoxycholic acid/熊去氧胆酸(10 μM;24 小时)可降低不同细胞类型中的 SARS-CoV-2 感染,并降低初级气道和受损类器官中的 ACE2 和 SHP 水平 [4]。
FXR在体外调节病毒感染[4] 我们的结果表明,用临床批准的药物Ursodeoxycholic acid/熊去氧胆酸或非处方药ZGG抑制FXR信号传导,可以降低多种细胞类型中ACE2的表达,Ursodeoxycholic acid/熊去氧胆酸是原发性胆汁性胆管炎(PBC)的一线治疗药物。为了考虑这一发现与新冠肺炎的相关性,我们研究了FXR介导的ACE2下调是否可以降低体外对SARS-CoV-2感染的易感性。为此,我们将胆囊胆管细胞、气道和肠道类器官暴露于生理水平的CDCA,以模拟体内存在的FXR激活的基线水平,并在UDCA或ZGG存在或不存在的情况下,用从患者鼻咽拭子中分离出的严重急性呼吸系统综合征冠状病毒2型感染它们(图1e、f)。用UDCA或ZGG抑制FXR信号传导可减少所有三种类器官中的病毒感染(图1e、f和扩展数据图5a)。然后,我们研究了观察到的病毒感染减少是否是FXR介导的ACE2下调的直接结果。首先,我们发现,使用shRNAs敲除FXR会降低ACE2的表达,并抑制胆管细胞类器官中的病毒感染,而与CDCA或UDCA或ZGG的存在无关(扩展数据图4d)。因此,在敲除后,用UDCA或ZGG治疗对病毒感染没有影响(扩展数据图4d)。接下来,为了确定ACE2的调节是否是UDCA和ZGG减少严重急性呼吸系统综合征冠状病毒2感染的唯一机制,我们用UDCA或ZGG处理了HEK293T细胞,这些细胞经过基因工程改造,能够独立于FXR过表达ACE2(扩展数据图6a,b),然后用严重急性呼吸系综合征冠状病毒-2感染它们。正如预期的那样,在没有ACE2调节的情况下,UDCA和ZGG不影响病毒复制(扩展数据图6c)。这些结果共同证实,UDCA和ZGG通过FXR介导的ACE2调节,在体外降低了多种细胞类型对严重急性呼吸系统综合征冠状病毒2型感染的易感性。 为了进一步探讨次级胆汁酸抑制肝脏FATP的生理和药理学意义,我们测试了Ursodeoxycholic acid/熊去氧胆酸/UDCA对原代肝细胞摄取LCFA的影响。使用基于FACS的LCFA摄取测定法,允许对活细胞进行门控,我们发现UDCA而不是TUDCA抑制了原代人肝细胞对LCFA的摄取(见图5)。UDCA还抑制了C57Bl/6动物原代小鼠肝细胞对LCFA的摄取,没有任何可检测到的细胞毒性作用(图5A右)。重要的是,这种作用完全依赖于FATP5,因为UDCA未能抑制FATP5缺失动物原代肝细胞对LCFA的摄取(图5A)。正如我们稳定的细胞系结果所预测的那样,次级胆汁酸DCA显著抑制了肝细胞对LCFA的摄取(图5B),而LCA对原代小鼠肝细胞对LCMA的摄取没有抑制作用(图5D)。此外,DCA介导的抑制与毒性无关(图5B右),重要的是,主要依赖于FATP5,因为这种作用在FATP5缺失的肝细胞中被消除(图5B)。我们使用14C标记的油酸盐证实DCA抑制了原代肝细胞对脂肪酸的摄取(补充图6)。随后的检测侧重于原代肝细胞对放射性标记代谢物的摄取,并证明DCA可以抑制多种脂肪酸的摄取,而不影响2-脱氧-D-[3H]葡萄糖/葡萄糖混合物的摄取(图5C)[5]。 |
| 体内研究 (In Vivo) |
在 C57BL/6J 野生型小鼠中,熊去氧胆酸(50、150 和 450 mg/kg;途径);每天一次,持续 21 天——导致体重减轻 [1]。在小鼠和仓鼠中,熊去氧胆酸(1% w/w 或 416 mg/kg;端口;7 天)可降低 ACE2 表达 [4]。在仓鼠中,熊去氧胆酸(416 mg/kg;侧壁;7 天)可有效减少 SARS-CoV-2 感染 [4]。
熊去氧胆酸(市售熊二醇)是一种天然胆汁酸,用于治疗各种肝脏和胃肠道疾病。Ursodiol可以调节胆汁酸库,这有可能改变肠道微生物群落结构。反过来,肠道微生物群落可以调节胆汁酸库,从而突出了肠道微生物群胆汁酸宿主轴的相互联系。尽管存在这些相互作用,但尚不清楚外源性施用熊二醇是否以及如何塑造传统小鼠的肠道微生物群落结构和胆汁酸库。本研究旨在描述熊二醇如何改变传统小鼠的胃肠道生态系统。C57BL/6J野生型小鼠经口灌胃给予三种剂量的熊二醇(50、150或450mg/kg/天)中的一种,持续21天。检查了肠道微生物群和胆汁酸的变化,包括粪便、回肠和盲肠内容物。血清中胆汁酸也进行了测定。用低剂量和高剂量熊二醇治疗的小鼠体重明显减轻。与预处理相比,回肠和盲肠内容物中的微生物群落结构和胆汁酸池发生了变化,在熊二醇治疗21天后,粪便中的微生物群结构和胆汁酸池也发生了纵向变化。在回肠和盲肠内容物中,Lachnospiraceae家族的成员对观察到的变化做出了重大贡献。这项研究首次全面阐述了外源性熊二醇如何塑造传统小鼠的健康胃肠道生态系统。进一步研究这些变化如何反过来改变宿主的生理反应非常重要。[1] 关于原发性胆汁性肝硬化(PBC)患者的死亡率和恶性肿瘤风险,以及使用Ursodeoxycholic acid/熊去氧胆酸是否会降低这种风险,存在争议。为了调查这个问题,我们从英国全科医学研究数据库中确定了930名PBC患者和9202名对照受试者。我们将常规熊去氧胆酸分为6个或更多处方的治疗和少于6个处方的非常规治疗。我们发现,与普通人群相比,PBC队列的死亡率增加了2.7倍[调整后的风险比(HR)为2.69;95%置信区间为2.35-3.09]。在服用常规熊去氧胆酸(43%)的患者中,死亡率增加了2.2倍(HR,2.19;95%CI,1.66-2.87),在未接受治疗的患者中增加了2.7倍(HR;2.69;95%CI2.18-3.33)。熊去氧胆酸治疗组的疾病不那么严重,这并不能解释死亡率的明显降低。经熊去氧胆酸治疗的患者患原发性肝癌的风险增加了3倍(HR,3.17;95%CI,0.64-15.62),而未经治疗的患者则增加了8倍(HR(7.77);95%CI(1.30-46.65))。 结论:我们发现,与普通人群相比,PBC患者的死亡率增加了3倍,定期使用熊去氧胆酸治疗后,死亡率有所降低。然而,熊去氧胆酸的观察效果没有统计学意义。[2] 众所周知,胆汁酸在吸收疏水性营养物质方面作为洗涤剂和在调节代谢方面作为信号分子发挥着重要作用。我们测试了天然胆汁酸干扰蛋白质介导的肝脏长链游离脂肪酸(LCFA)摄取的新假设。为此,将表达脂肪酸转运蛋白的稳定细胞系以及来自小鼠和人类肝脏的原代肝细胞与原代和继发胆汁酸一起孵育,以确定其对LCFA摄取率的影响。我们确定Ursodeoxycholic acid/熊去氧胆酸(UDCA)和脱氧胆酸(DCA)是肝脏特异性脂肪酸转运蛋白5(FATP5)的两种最有效的抑制剂。UDCA和DCA都能够以FATP5依赖的方式抑制原代肝细胞对LCFA的摄取。随后,在体内用这些次生胆汁酸处理小鼠,以评估其抑制饮食诱导的肝甘油三酯积聚的能力。通过注射或作为高脂肪饮食的一部分在体内施用DCA显著抑制了肝脏脂肪酸的摄取,并将肝脏甘油三酯降低了50%以上。 结论:这些数据表明,特定胆汁酸,特别是次级胆汁酸DCA,在调节肝脏LCFA摄取中起着新的作用。这些结果阐明了一种以前未被重视的方法,即特定的胆汁酸,如UDCA和DCA,可以影响肝脏甘油三酯代谢,并可能导致对抗肥胖相关脂肪肝疾病的新方法[5]。 |
| 酶活实验 |
FXR活性的调节[4]
CDCA、ZGG和UDCA/熊去氧胆酸购自xxx,并按照制造商的说明重新配制。为了调节FXR活性,将类器官与终浓度为10μM的CDCA或10μM CDCA与10μM UDCA或ZGG联合孵育。 |
| 细胞实验 |
ChIP[4]
每个ChIP大约使用6×106个细胞,在收集前2小时,将细胞与含有100μM CDCA、UDCA/熊去氧胆酸或ZGG的新鲜培养基一起孵育。根据制造商的说明,使用True Micro ChIP试剂盒进行ChIP。简而言之,在预清除后,将裂解物与FXR抗体(补充表1)或非免疫IgG一起孵育过夜。完成ChIP,并使用MicroChip DiaPure柱纯化免疫沉淀的DNA。如前所述,使用ΔΔCt方法通过qPCR对样本进行分析51(引物序列见补充表3)。众所周知的FXR靶基因OSTα(也称为SLC51A;参考文献54)上FXRE侧翼的引物用作阳性对照,而ACE2启动子上远离FXRE的位点侧翼的引物则用作阴性对照。结果标准化为非免疫IgG-ChIP对照观察到的富集。 萤光素酶记者[4] 使用人类基因组DNA作为模板扩增ACE2基因和SHP基因(也称为NR0B2)中含有FXRE IR-1的两个不同片段,并将其插入pGL3启动子荧光素酶载体上。ACE2和SHP IR-1突变体是使用定点突变方法产生的。所用引物的序列见补充表4。使用TransIT-293转染试剂将这些基因报告构建体与市售的FXR表达质粒共转染到HEK293细胞中。转染后24小时,细胞在新鲜培养基中用50μM的CDCA、UDCA/Ursodeoxycholic acid/熊去氧胆酸和ZGG处理8小时 h.用GLO萤光素酶报告物测定系统测定萤光素酶活性,并将值归一化到空的pGL3载体上。 细胞毒性和存活率[4] 用0.1μM–100μM的CDCA、UDCA/熊去氧胆酸或ZGG处理初级类器官,并使用台盼蓝和Countess II细胞计数器计数活细胞的百分比。在SpectraMax M2上使用SoftMax Pro 5.4.4,使用基于刃天青的PrestoBlue测定法测量用10μM CDCA、UDCA或ZGG处理的初级类器官的细胞存活率。 严重急性呼吸系统综合征冠状病毒2型复制的萤光素酶报告基因[4] 如前所述,在病毒复制过程中产生了用于检测严重急性呼吸系统综合征冠状病毒2型蛋白酶活性的萤光素酶报告基因28。简而言之,将稳定表达ACE2、肾荧光素酶(Rluc)和严重急性呼吸系综合征冠状病毒-2木瓜蛋白酶样蛋白酶可激活的圆形置换萤火虫萤光素酶(FFluc)的HEK293T报告细胞接种在平底96孔板中。第二天早上,用指定剂量的CDCA、UDCA/熊去氧胆酸和ZGG处理细胞,并以0.01的MOI感染严重急性呼吸系统综合征冠状病毒2型。将严重急性呼吸综合征冠状病毒2型RdRp抑制剂瑞德西韦和阻断严重急性呼吸系统综合征冠状病毒-2刺突与ACE2(REGN-COV2)相互作用的中和抗体混合物作为阳性对照。24小时后,细胞在用PBS和1%NP-40 1:1稀释的双Glo萤光素酶缓冲液中裂解。然后将裂解物转移到不透明的96孔板上,根据制造商的说明,使用Dual-Glo试剂盒测量病毒复制,以FFluc/Rluc活性的比率进行定量。FFluc/Rluc比值表示为最大值的分数,然后使用GraphPad Prism中的Sigmoid、4PL、X is log(浓度)函数进行分析。 |
| 动物实验 |
Animal/Disease Models: 5weeks old C57BL/6J WT mice (male and female) [1]
Doses: 50, 150 and 450 mg/kg dissolved in corn oil Route of Administration: po (oral gavage); one time/day for 21 days Experimental Results: Mice in the 50 mg/kg and 450 mg/kg groups continued to lose significant weight within a week. At the 50 mg/kg dose, this weight loss persisted throughout the experiment. At the 450 mg/kg dose, weight loss was initially noted during the first and third weeks of ursodiol administration. At the 150 mg/kg dose, there was no significant difference in body weight compared to untreated mice. Animal/Disease Models: FVB/N mice and Syrian golden hamsters [4] Doses: 1% w/w for mice, 416 mg/kg for hamsters. Route of Administration: feed or po (oral gavage), 7 days. Experimental Results: ACE2 expression diminished. Animal/Disease Models: Syrian golden hamster, SARS-CoV-2 infection model [4] Doses: 416 mg/kg Route of Administration: po (oral gavage), 7 days Experimental Results: n = 6 of 9 sentinel animals prevented SARS -Transmission of CoV-2 (33% infected vs. 67% uninfected). Ursodiol/Ursodeoxycholic acid dosing experiment and sample collection [1] Groups of 5 week old C57BL/6J WT mice (male and female) were treated with ursodiol/Ursodeoxycholic acid at three distinct doses (50, 150, and 450 mg/kg dissolved in corn oil) given daily via oral gavage for 21 day. These distinct doses were selected for a proof of concept experiment in order to achieve sufficient intestinal concentrations of ursodiol to alter the life cycle of Clostridioides difficile in vivo. The total volume gavaged was consistent between the three distinct doses in order to control for the volume of corn oil administered. Ursodiol dosing was adjusted once weekly, based on current weight. Two independent experiments were performed, with a total of n = 8 mice (female/male) per treatment group. Mice were weighed daily over the course of the experiment. Fecal pellets were collected twice daily, flash-frozen and stored at -80°C until further analysis. A control group of mice were necropsied prior to initiating any treatments (pretreatment group). This pretreatment group serves as a microbiome and bile acid metabolome baseline prior to mice receiving ursodiol treatment. An additional control group of mice underwent daily handling similar to the treatment groups, but were not administered ursodiol (no treatment control). Necropsy was performed at day 21 in all ursodiol treated mice and the no treatment control mice. Gastrointestinal contents and tissue from the ileum and cecum were collected, flash frozen in liquid nitrogen, and stored at -80°C until further analysis. Serum and bile aspirated from the gallbladder was obtained flash frozen in liquid nitrogen, and stored at -80°C until further analysis. Mice [4] Mice were housed in a 12 h–12 h dark–light cycle, with a humidity of 45–65% and temperature of 20–24 °C. Age-matched female mice were used. Mice were assigned randomly to treatment and control groups. Mice in the treatment group received chow supplemented with 1% w/w UDCA/Ursodeoxycholic acid and 1% w/w cholic acid, whereas mice in the control group received chow supplemented with 1% w/w cholic acid58. Cholic acid was used to activate FXR and study the effects of UDCA on FXR activation19. The mice were fed ad libitum for seven days. Data were analysed blinded to the identity of the experimental groups. Hamsters [4] Golden Syrian hamsters were purchased from Janvier Labs. Hamsters were housed in a 12 h–12 h dark–light cycle, with a humidity of 45–65% and temperature of 20–24 °C. Age-matched male hamsters were used, weighing between 80 g and 100 g. Hamsters were assigned randomly to treatment and control groups. Hamsters in the treatment groups received a daily oral regimen of UDCAUrsodeoxycholic acid (416 mg per kg) by oral gavage, whereas those in the control group received vehicle only. The hamsters were fed ad libitum and treatment continued for seven days to achieve a similar blood concentration of UDCA to that observed in patients taking UDCA29 (Extended Data Fig. 9a). [4] For testing the effects of Ursodeoxycholic acid/UDCA against SARS-CoV-2 infection, one hamster was directly inoculated by the intranasal route with 1 × 102 plaque-forming units (PFU) in 100 µl PBS. Each infected hamster was placed on one side of a transmission cage. The cage was divided with an aerated barrier that allowed the infected hamster to be co-housed with previously treated uninfected hamsters housed on the other side, permitting us to study viral infection by aerosol transmission. Daily swabs were collected from all hamsters to monitor the infection by qPCR for the viral N gene. On day 4 after infection, the hamsters were euthanized and lungs and nasal turbinates were collected for quantification of viral infection. The experiment was repeated n = 3 times for a total of n = 9 UDCA and n = 6 vehicle hamsters. Data were analysed blinded to the identity of the experimental groups. Animal Experiments [5] The generation of FATP5 null mice has been described previously Mice were fed standard chow prior to the high-fat diet experiments (60% calories from fat). All experiments were performed with individually housed animals. For the bile acid injection experiments mice were injected subcutaneously with 3.2mg/kg DCA or LCA in 20 μl DMSO on the back above the right hip once a day for 7 weeks while consuming a high-fat diet. For bile acid feeding experiments 5mg/g Ursodeoxycholic acid/UDCA, 0.5mg/g DCA or 0.5mg/g LCA in high fat food were mixed. The mice were fed the supplemented high fat diet for 7 weeks. Their food intake and body weight were recorded once a week. During this time, the mice had ad libitum access to water and high fat food. For tissue and plasma collection, mice were fasted 4 hours prior to euthanization. After euthansia by CO2 asphyxiation, subcutaneous injection sites were examined to verify that no bile acid precipitates had accumulated. Liver and other organs were removed, lysed and the protein and TAG concentrations of organ lysates were assayed using the BCA protein assay kit and infinity TAG kit respectively. All procedures were approved by the UC Berkeley ACUC. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Normally, endogenous ursodeoxycholic acid constitutes a minor fraction (about 5%) of the total human bile acid pool. Following oral administration, the majority of ursodiol is absorbed by passive diffusion, and its absorption is incomplete. Once absorbed, ursodiol undergoes hepatic extraction to about 50% in the absence of liver disease. As the severity of liver disease increases, the extent of extraction decreases. During chronic administration of ursodiol, it becomes a major biliary and plasma bile acid. At a chronic dose of 13 to 15 mg/kg/day, ursodiol constitutes 30-50% of biliary and plasma bile acids. Ursodeoxycholic acid is excreted primarily in the feces. Renal elimination is a minor elimination pathway. With treatment, urinary excretion increases but remains less than 1% except in severe cholestatic liver disease. The volume of distribution of ursodeoxycholic acid (UDCA) has not been determined; however, it is expected to be small since UDCA is mostly distributed in the bile in the gallbladder and small intestines. Metabolism / Metabolites Upon administration, ursodeoxycholic acid (UDCA) enters the portal vein and into the liver, where it undergoes conjugation with glycine or taurine. UDCA is also decreased into bile. Glycine or taurine conjugates are absorbed in the small intestine via passive and active mechanisms. The conjugates can also be deconjugated in the ileum by intestinal enzymes, leading to the formation of free UDCA that can be reabsorbed and re-conjugated in the liver. Nonabsorbed UDCA passes into the colon, where it undergoes 7-dehydroxylation by intestinal bacteria to lithocholic acid. Some UDCA is epimerized to [chenodeoxycholic acid] via a 7-oxo intermediate. Chenodeoxycholic acid also undergoes 7-dehydroxylation to form lithocholic acid. These metabolites are poorly soluble and excreted in the feces. A small portion of lithocholic acid is reabsorbed, conjugated in the liver with glycine or taurine, and sulfated at the 3 position. The resulting sulfated lithocholic acid conjugates are excreted in bile and then lost in feces. Biological Half-Life The estimated half-life ranges from 3.5 to 5.8 days. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
n multiple clinical trials in a variety of conditions, ursodiol has not been found to cause increases in serum enzyme elevations, worsening of underlying liver disease or clinically apparent liver injury. Nevertheless, there have been rare reports of clinical decompensation in patients with advanced liver disease and cirrhosis started on ursodiol, but the reason for such reactions is not known. In at least one instance, there was recurrence of jaundice on restarting ursodiol. Thus, ursodiol has beneficial effects on several forms of liver disease and has not been convincingly linked to cases of clinically apparent acute liver injury in patients without cirrhosis. There is some concern that ursodiol may be harmful in patients with advanced liver disease (Childs class B and C) and such patients probably should not receive ursodiol. Likelihood score: D (possible rare cause of acute decompensation of preexisting liver disease). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Ursodiol is naturally present in human milk. Because of the low levels of ursodiol (ursodeoxycholic acid) in breastmilk after exogenous administration, amounts ingested by the infant are small and are not expected to cause any adverse effects in breastfed infants. Ursodiol has been given directly to newborns to safely and successfully treat prolonged neonatal jaundice. No special precautions are required. ◉ Effects in Breastfed Infants One breastfed (extent not stated) infant developed normally over the first 6 months of life during maternal ursodiol therapy of 750 to 1000 mg daily. Seven women who were taking ursodiol 14 mg/kg daily near term and postpartum. They reported no adverse reactions in their breastfed infants during the early postpartum period. A mother receiving oral ursodiol 250 mg 3 times daily for primary biliary cirrhosis reportedly breastfed her infant normally, although the extent and duration of breastfeeding was not stated. A woman with primary biliary cirrhosis developed severe pruritus and elevated serum bile acids 3 weeks postpartum. Ursodiol was started at a dose of 500 mg (7.5 mg/kg) daily, increasing to 1500 mg (25 mg/kg) daily over the next 8 weeks. Psychomotor development of her breastfed (extent not stated) infant was normal, and no apparent side effects were observed in the infant. A retrospective review of the medical records of pregnant patients at a hospital in Ankara, Türkiye who had a diagnosis of primary biliary cirrhosis found 8 patients who took ursodiol postpartum in doses of 13–15 mg/kg daily. “Most” of the patients breastfed their infants (extent not stated). No infant side effects were reported. A woman was breastfeeding her 8-day-old preterm infant 10 times daily for about 15 minutes each time. The infant was born by cesarean section at 34 weeks of gestation with a weight of 3600 grams. She was diagnosed with cholestasis, type 1 diabetes, and hypothyroidism. She was treated with ursodiol 500 mg daily, insulin levemir and aspart, and levothyroxine. She was also taking cefuroxime, flurbiprofen, a combination of acetaminophen, propyphenazone, and caffeine. The mother took the ursodiol for a total of 12 days, cefuroxime and the analgesic combination for 10 days and flurbiprofen for 15 days. No adverse effects were noticed during the period of ursodiol treatment. Twenty nursing mothers were taking ursodiol for cholestasis in daily dosages of 500 to 1500 mg or 13 to 15 mg/kg, depending on the condition. Ursodiol was discontinued 3 days postpartum. No apparent side effects were observed in any newborn infant based on standard clinical examination during early postnatal period, and no deterioration in postnatal development was observed during routine 1-year follow-up on routine pediatric examinations. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Unconjugated ursodeoxycholic acid is at least 70% bound to plasma proteins in health individuals. There is no information regarding the protein binding of conjugated ursodeoxycholic acid. Toxicity Summary UDCA has been shown to have potentially toxic molecular properties. UDCA breaks down into toxic lithocholic acid. After being absorbed in the small intestine, UDCA undergoes hepatic conjugation. Beyond conjugation, UDCA does not experience further breakdown by the liver or intestinal mucosa. It becomes oxidized or reduced, yielding either 7-keto-lithocholic acid or lithocholic acid. Litcholic acid can be toxic to liver cells and even cause liver failure in those with compromised sulfation. It also leads to segmental bile duct injury, hepatocyte failure, and death. |
| 参考文献 | |
| 其他信息 |
Pharmacodynamics
Ursodeoxycholic acid (UDCA) is a secondary bile acid with cytoprotectant, immunomodulating, and choleretic effects. It reduces the cholesterol fraction of biliary lipids. UDCA inhibits the absorption of cholesterol in the intestine and the secretion of cholesterol into bile, decreasing biliary cholesterol saturation. UDCA increases bile acid flow and promotes the secretion of bile acids. Ursodeoxycholic acid is a bile acid found in the bile of bears (Ursidae) as a conjugate with taurine. Used therapeutically, it prevents the synthesis and absorption of cholesterol and can lead to the dissolution of gallstones. It has a role as a human metabolite and a mouse metabolite. It is a bile acid, a dihydroxy-5beta-cholanic acid and a C24-steroid. It is a conjugate acid of an ursodeoxycholate. Ursodeoxycholic acid (UDCA), also known as ursodiol, is a naturally-occurring bile acid that constitutes a minor fraction of the human bile acid pool. UDCA has been used to treat liver disease for decades: its first use in traditional medicine dates back more than a hundred years. UDCA was first characterized in the bile of the Chinese black bear and is formed by 7b-epimerization of [chenodeoxycholic acid], which is a primary bile acid. Due to its hydrophilicity, UDCA is less toxic than [cholic acid] or [chenodeoxycholic acid]. UDCA was first approved by the FDA in 1987 for dissolution of gallstones and for primary biliary cirrhosis in 1996. UDCA works by replacing the hydrophobic or more toxic bile acids from the bile acid pool. Ursodiol is a Bile Acid. Ursodeoxycholic acid or ursodiol is a naturally occurring bile acid that is used dissolve cholesterol gall stones and to treat cholestatic forms of liver diseases including primary biliary cirrhosis. Ursodiol has been linked to rare instances of transient and mild serum aminotransferase elevations during therapy and to rare instances of jaundice and worsening of liver disease in patients with preexisting cirrhosis. Ursodeoxycholic acid has been reported in Myocastor coypus with data available. LOTUS - the natural products occurrence database Ursodiol is a synthetically-derived form of ursodiol, a bile acid produced by the liver and secreted and stored in the gallbladder. Also produced by the Chinese black bear liver, ursodiol has been used in the treatment of liver disease for centuries. This agent dissolves or prevents cholesterol gallstones by blocking hepatic cholesterol production and decreasing bile cholesterol. Ursodiol also reduces the absorption of cholesterol from the intestinal tract. URSODIOL is a small molecule drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 1987 and is indicated for primary biliary cirrhosis and biliary liver cirrhosis and has 25 investigational indications. An epimer of chenodeoxycholic acid. It is a mammalian bile acid found first in the bear and is apparently either a precursor or a product of chenodeoxycholate. Its administration changes the composition of bile and may dissolve gallstones. It is used as a cholagogue and choleretic. Ursodeoxycholic acid (commercially available as ursodiol) is a naturally occurring bile acid that is used to treat a variety of hepatic and gastrointestinal diseases. Ursodiol can modulate bile acid pools, which have the potential to alter the gut microbiota community structure. In turn, the gut microbial community can modulate bile acid pools, thus highlighting the interconnectedness of the gut microbiota-bile acid-host axis. Despite these interactions, it remains unclear if and how exogenously administered ursodiol shapes the gut microbial community structure and bile acid pool in conventional mice. This study aims to characterize how ursodiol alters the gastrointestinal ecosystem in conventional mice. C57BL/6J wildtype mice were given one of three doses of ursodiol (50, 150, or 450 mg/kg/day) by oral gavage for 21 days. Alterations in the gut microbiota and bile acids were examined including stool, ileal, and cecal content. Bile acids were also measured in serum. Significant weight loss was seen in mice treated with the low and high dose of ursodiol. Alterations in the microbial community structure and bile acid pool were seen in ileal and cecal content compared to pretreatment, and longitudinally in feces following the 21-day ursodiol treatment. In both ileal and cecal content, members of the Lachnospiraceae Family significantly contributed to the changes observed. This study is the first to provide a comprehensive view of how exogenously administered ursodiol shapes the healthy gastrointestinal ecosystem in conventional mice. Further studies to investigate how these changes in turn modify the host physiologic response are important.[1] Ursodeoxycholic acid is currently the only established drug for the treatment of chronic cholestatic liver diseases. It has cytoprotective, anti-apoptotic, membrane stabilizing, anti-oxidative and immunomodulatory effects. Prolonged administration of ursodeoxycholic acid in patients with primary biliary cirrhosis (PBC) is associated with survival benefit and a delaying of liver transplantation. There is evidence that it might even prevent progression of the histologic stage of PBC. It also has a beneficial effect on primary sclerosing cholangitis, intrahepatic cholestasis of pregnancy, liver disease associated with cystic fibrosis, chronic graft versus host disease, total parenteral nutrition associated cholestasis and various pediatric cholestatic liver diseases. In the present review the current knowledge about the mechanisms of the action and role of ursodeoxycholic acid in the treatment of various liver diseases has been discussed. [3] Preventing SARS-CoV-2 infection by modulating viral host receptors, such as angiotensin-converting enzyme 2 (ACE2)1, could represent a new chemoprophylactic approach for COVID-19 that complements vaccination2,3. However, the mechanisms that control the expression of ACE2 remain unclear. Here we show that the farnesoid X receptor (FXR) is a direct regulator of ACE2 transcription in several tissues affected by COVID-19, including the gastrointestinal and respiratory systems. We then use the over-the-counter compound z-guggulsterone and the off-patent drug ursodeoxycholic acid (UDCA) to reduce FXR signalling and downregulate ACE2 in human lung, cholangiocyte and intestinal organoids and in the corresponding tissues in mice and hamsters. We show that the UDCA-mediated downregulation of ACE2 reduces susceptibility to SARS-CoV-2 infection in vitro, in vivo and in human lungs and livers perfused ex situ. Furthermore, we reveal that UDCA reduces the expression of ACE2 in the nasal epithelium in humans. Finally, we identify a correlation between UDCA treatment and positive clinical outcomes after SARS-CoV-2 infection using retrospective registry data, and confirm these findings in an independent validation cohort of recipients of liver transplants. In conclusion, we show that FXR has a role in controlling ACE2 expression and provide evidence that modulation of this pathway could be beneficial for reducing SARS-CoV-2 infection, paving the way for future clinical trials.[4] Bile acids are known to play important roles as detergents in the absorption of hydrophobic nutrients and as signaling molecules in the regulation of metabolism. We tested the novel hypothesis that naturally occurring bile acids interfere with protein-mediated hepatic long chain free fatty acid (LCFA) uptake. To this end, stable cell lines expressing fatty acid transporters as well as primary hepatocytes from mouse and human livers were incubated with primary and secondary bile acids to determine their effects on LCFA uptake rates. We identified ursodeoxycholic acid (UDCA) and deoxycholic acid (DCA) as the two most potent inhibitors of the liver-specific fatty acid transport protein 5 (FATP5). Both UDCA and DCA were able to inhibit LCFA uptake by primary hepatocytes in a FATP5-dependent manner. Subsequently, mice were treated with these secondary bile acids in vivo to assess their ability to inhibit diet-induced hepatic triglyceride accumulation. Administration of DCA in vivo via injection or as part of a high-fat diet significantly inhibited hepatic fatty acid uptake and reduced liver triglycerides by more than 50%. Conclusion: The data demonstrate a novel role for specific bile acids, and the secondary bile acid DCA in particular, in the regulation of hepatic LCFA uptake. The results illuminate a previously unappreciated means by which specific bile acids, such as UDCA and DCA, can impact hepatic triglyceride metabolism and may lead to novel approaches to combat obesity-associated fatty liver disease.[5] |
| 分子式 |
C24H40O4
|
|---|---|
| 分子量 |
392.5720
|
| 精确质量 |
392.292
|
| 元素分析 |
C, 73.43; H, 10.27; O, 16.30
|
| CAS号 |
128-13-2
|
| 相关CAS号 |
Ursodeoxycholic acid sodium;2898-95-5
|
| PubChem CID |
31401
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
547.1±25.0 °C at 760 mmHg
|
| 熔点 |
203-206 ºC
|
| 闪点 |
298.8±19.7 °C
|
| 蒸汽压 |
0.0±3.3 mmHg at 25°C
|
| 折射率 |
1.543
|
| LogP |
4.66
|
| tPSA |
77.76
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
605
|
| 定义原子立体中心数目 |
10
|
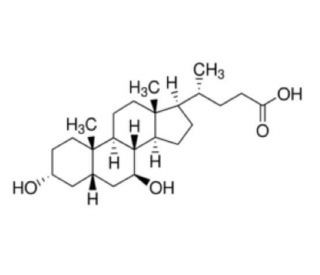
| SMILES |
C[C@H](CCC(=O)O)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2[C@H](C[C@H]4[C@@]3(CC[C@H](C4)O)C)O)C
|
| InChi Key |
RUDATBOHQWOJDD-UZVSRGJWSA-N
|
| InChi Code |
InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20+,22+,23+,24-/m1/s1
|
| 化学名 |
(4R)-4-[(3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid
|
| 别名 |
URSODEOXYCHOLIC ACID; ursodiol; 128-13-2; Actigall; UDCA; Ursodesoxycholic acid; Urso Forte; Litursol;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 100 mg/mL (~254.73 mM)
H2O : ~1 mg/mL (~2.55 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.30 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.30 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (5.30 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5473 mL | 12.7366 mL | 25.4732 mL | |
| 5 mM | 0.5095 mL | 2.5473 mL | 5.0946 mL | |
| 10 mM | 0.2547 mL | 1.2737 mL | 2.5473 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Fenofibrate in Patients with Primary Biliary Cholangitis (PBC)
CTID: NCT06365424
Phase: Phase 2/Phase 3 Status: Recruiting
Date: 2024-09-19