| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Retinoid X receptor (RXR)
|
|---|---|
| 体外研究 (In Vitro) |
UVI3003 抑制非洲爪蟾和人 RXRα 的活性,IC50 分别为 0.22 和 0.24 μM。 UVI3003 对 hPPARγ 和 mPPARγ 几乎无效,但完全激活 xPPARγ,EC50 为 12.6 μM [1]。由眼外肌 (EOM) 或肺上皮 (LEG) 生成的 EECD34 细胞的生长速率不受 UVI 3003 (10 μM) 的影响。结蛋白表达和 EECD34 细胞融合对 UVI 3003 的反应有 65.4% 的差异 [2]。
|
| 体内研究 (In Vivo) |
UVI3003诱导热带爪蟾胚胎多发畸形[1]
在不同发育窗口期暴露于UVI3003会导致明显的发育迟缓和多种畸形(图1)。UVI3003处理组最常见的表型是前额缩小、晶状体混浊和鳍窄。在所有NF10-19处理组和NF19-25高剂量组中,均观察到直乳伸长,而在暴露后期窗口中,直乳表型增大。UVI3003在NF - 10-25期致畸能力较弱,在NF - 25-39期致畸能力显著增强,在NF - 39-43期致畸能力显著降低。在所有NF 31-36、NF36-39处理组和nf39 -41中、高剂量组中,胚胎体长均显著减少(补充图3) UVI3003可下调热带爪蟾胚胎中PPARγ的表达[1] 在7个不同的uvi3003暴露窗口后,对编码RXRs及其异二聚体伙伴RARs、ppar和TRs的mrna表达进行了评估。我们发现RARβ在早期暴露期下调(图2B2),而RXRs, TRα和TRβ在胚胎发生后期处理后受到影响(图2A, D)。PPARγ的表达在所有处理期间都明显降低(图2C3)。我们在最敏感的暴露窗口测试了TPT处理的效果,发现PPARγ在高剂量下也被下调(图3)。因此,我们的结果表明UVI3003和TPT在热带天蛾胚胎中下调了PPARγ。 |
| 酶活实验 |
体外模型(cos7细胞)荧光素酶报告基因测定[1]
核受体配体结合域gal4 -人RXRα (Perlmann等人,1996)、-非洲爪蟾RXRα (Blumberg等人,1992)、-人PPARγ (Greene等人,1994)、-小鼠PPARγ (kleiewer等人,1994)的pCMX-GAL4质粒融合构建物此前被描述过(Chamorro-García等人,2012)。利用PCR方法从cDNA文库中分离出非洲爪蟾PPARγ,并将其克隆到pCMX-GAL4表达载体上,其克隆引物见补充表2。将1微克pCMX- gal4效应质粒与5 μg pCMX-β-半乳糖苷酶转染对照、5 μg tk-(MH100)4-荧光素酶报告基因和14 μg pUC19载体质粒(每96孔板)共转染至Cos7细胞(Sambrook and Russell, 2005)。UVI3003分别以10−5 M和10−4 M连续稀释3倍,用于RXRα拮抗和PPARγ激活试验。TPT从10- 6 M连续稀释10倍或3倍,用于RXRα和PPARγ活化试验。对照化合物HX531 (RXR拮抗剂)、IRX4204(以前指定为AGN194204和NRX194204, RXR激动剂)和ROSI(罗格列酮,PPARγ激动剂)在10- 5 M范围内以10倍的连续稀释度进行测试(Kanayasu-Toyoda等,2005;Vuligonda et al., 1996)。所有转染均为三次,并在多次实验中重复。 |
| 细胞实验 |
增殖实验。[2]
分选后的EECD34细胞分别镀于Matrigel包被皿8孔Permanox室载玻片或48孔板,密度为10,000个细胞/cm2,或15孔μ slide Angiogenesis板,密度为7680个细胞/cm2。每隔一天更换一次增殖介质,直到细胞达到适当的密度。 在~ 30-40%的合流条件下,细胞分别用乙醇、全反式维甲酸(10−6M)、RAR逆激动剂BMS493(10−5M)或RXR拮抗剂UVI3003(10−5M)在乙醇终浓度为0.1%的增殖培养基中处理24小时。在24小时处理结束时,评估细胞增殖率。 融合实验。[2] 在~ 70-80%汇合时,用乙醇处理细胞,全反式维甲酸(10−6 M), BMS493(10−5M),或UVI3003(10−5 M)在融合培养基(DMEM, 5%马血清,1%青霉素/链霉素)中72h,所有处理的最终浓度为0.1%的乙醇。48小时后更换含有载体、视黄酸、BMS493或UVI 3003的融合介质。72小时处理结束时,评估细胞融合情况。 |
| 动物实验 |
Exposure experiments using Xenopus tropicalis embryos[1]
Xenopus tropicalis adults were maintained according to previous methods (Yu et al., 2011). Breeding was induced by subcutaneous injection of human chorionic gonadotrophin (hCG) as described (Yu et al., 2011; Hu et al., 2015a). The exposure experiments were conducted following the Frog Embryo Teratogenesis Assay (FETAX) protocol (Fort and Paul, 2002) with some modifications. Briefly, approximately 12 h after the second injection of hCG, adults were removed from their tanks, and embryos were harvested without removing the jelly coats (Supplementary Fig. 2). UVI3003 was dissolved in DMSO and then diluted into FETAX medium. Four replicate dishes (n = 4) were used in each control or treatment group of 20 embryos for morphological observations and real-time quantitative PCR analysis.[1] The EC50 of UVI3003 is 0.5 μM after 48 hrs treatment from NF10 in X. tropicalis embryos (Zhu et al., 2014). In this study, we chose 1, 1.5, 2 μM of UVI3003 to treat embryos in short exposure windows (6-8.5 hrs) from gastrulation (Nieuwkoop and Faber stage 10) to larval stage (NF43). 10 embryos were collected immediately after the exposure windows ended for real-time quantitative PCR analysis; the other 10 embryos were rinsed with FETAX medium three times and maintained at 26 ± 0.5°C in the dark for later morphological analysis. All exposure experiments ended when the control embryos reached NF43. To minimize biological variation, embryos for each exposure window were chosen from one pair of frogs. Luciferase reporter assay using in vivo model (Xenopus laevis embryos)[1] Xenopus laevis eggs were fertilized in vitro as described previously (Janesick et al., 2012), and embryos were staged according to Nieuwkoopand Faber (Nieuwkoop and Faber, 1956). Embryos were microinjected at the 2- or 4-cell stage with 50 pg/embryo pCMX-GAL4-xPPARγ mRNAor β-galactosidase (control) mRNA together with 50 pg/embryo tk-(MH100)4-luciferase reporter DNA. Microinjected embryos were treated at stage 8 with the following chemicals (in 0.1× MBS): UVI3003 (1, 5, 10 μM), TPT (0.01, 0.05, 0.1 μM), TBT (RXR and PPARγ agonist, 0.05 μM) or vehicle (0.1% DMSO). For each treatment, 25 embryos were treated in glass 60-mm Petri dishes containing 10 mL of MBS + chemical, and two replicate dishes were used for each concentration. Treated embryos were separated into five-embryo aliquots at neural stage for luciferase assays (Janesick et al., 2012, 2014). Each group of five embryos was considered one biological replicate. |
| 参考文献 | |
| 其他信息 |
The RXR agonist (triphenyltin, TPT) and the RXR antagonist (UVI3003) both show teratogenicity and, unexpectedly, induce similar malformations in Xenopus tropicalis embryos. In the present study, we exposed X. tropicalis embryos to UVI3003 in seven specific developmental windows and identified changes in gene expression. We further measured the ability of UVI3003 to activate Xenopus RXRα (xRXRα) and PPARγ (xPPARγ) in vitro and in vivo. We found that UVI3003 activated xPPARγ either in Cos7 cells (in vitro) or Xenopus embryos (in vivo). UVI3003 did not significantly activate human or mouse PPARγ in vitro; therefore, the activation of Xenopus PPARγ by UVI3003 is novel. The ability of UVI3003 to activate xPPARγ explains why UVI3003 and TPT yield similar phenotypes in Xenopus embryos. Our results indicate that activating PPARγ leads to teratogenic effects in Xenopus embryos. More generally, we infer that chemicals known to specifically modulate mammalian nuclear hormone receptors cannot be assumed to have the same activity in non-mammalian species, such as Xenopus. Rather they must be tested for activity and specificity on receptors of the species in question to avoid making inappropriate conclusions.[1]
One major difference between limb and extraocular muscles (EOM) is the presence of an enriched population of Pitx2-positive myogenic precursor cells in EOM compared to limb muscle. We hypothesize that retinoic acid regulates Pitx2 expression in EOM myogenic precursor cells and that its effects would differ in leg muscle. The two muscle groups expressed differential retinoic acid receptor (RAR) and retinoid X receptor (RXR) levels. RXR co-localized with the Pitx2-positive cells but not with those expressing Pax7. EOM-derived and LEG-derived EECD34 cells were treated with vehicle, retinoic acid, the RXR agonist bexarotene, the RAR inverse agonist BMS493, or the RXR antagonist UVI 3003. In vitro, fewer EOM-derived EECD34 cells expressed desmin and fused, while more LEG-derived cells expressed desmin and fused when treated with retinoic acid compared to vehicle. Both EOM and LEG-derived EECD34 cells exposed to retinoic acid showed a higher percentage of cells expressing Pitx2 compared to vehicle, supporting the hypothesis that retinoic acid plays a role in maintaining Pitx2 expression. We hypothesize that retinoic acid signaling aids in the maintenance of large numbers of undifferentiated myogenic precursor cells in the EOM, which would be required to maintain EOM normalcy throughout a lifetime of myonuclear turnover.[2] |
| 分子式 |
C28H36O4
|
|---|---|
| 分子量 |
436.5830
|
| 精确质量 |
436.261
|
| 元素分析 |
C, 77.03; H, 8.31; O, 14.66
|
| CAS号 |
847239-17-2
|
| 相关CAS号 |
847239-17-2;
|
| PubChem CID |
44566108
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
7.075
|
| tPSA |
66.76
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
32
|
| 分子复杂度/Complexity |
652
|
| 定义原子立体中心数目 |
0
|
| SMILES |
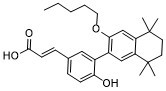
CCCCCOC1=CC2=C(C=C1C3=C(C=CC(=C3)/C=C/C(=O)O)O)C(CCC2(C)C)(C)C
|
| InChi Key |
APJSHECCIRQQDV-ZRDIBKRKSA-N
|
| InChi Code |
InChI=1S/C28H36O4/c1-6-7-8-15-32-25-18-23-22(27(2,3)13-14-28(23,4)5)17-21(25)20-16-19(9-11-24(20)29)10-12-26(30)31/h9-12,16-18,29H,6-8,13-15H2,1-5H3,(H,30,31)/b12-10+
|
| 化学名 |
(E)-3-[4-hydroxy-3-(5,5,8,8-tetramethyl-3-pentoxy-6,7-dihydronaphthalen-2-yl)phenyl]prop-2-enoic acid
|
| 别名 |
UVI 3003; 847239-17-2; UVI3003; UVI-3003; (2E)-3-{4-hydroxy-3-[5,5,8,8-tetramethyl-3-(pentyloxy)-5,6,7,8-tetrahydronaphthalen-2-yl]phenyl}prop-2-enoic acid; compound 10e [PMID: 19408900]; compound 10e (PMID: 19408900); (E)-3-[4-hydroxy-3-(5,5,8,8-tetramethyl-3-pentoxy-6,7-dihydronaphthalen-2-yl)phenyl]prop-2-enoic acid;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~229.05 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.73 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.73 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2905 mL | 11.4527 mL | 22.9053 mL | |
| 5 mM | 0.4581 mL | 2.2905 mL | 4.5811 mL | |
| 10 mM | 0.2291 mL | 1.1453 mL | 2.2905 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。