| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
Endogenous Metabolite; PPARβ/δ (Kd = 17 nM); PPARα (Kd = 103 nM); PPARγ (Kd = 178 nM); PPARα (IC50 = 14 nM); PPARγ (IC50 = 14 nM); RARβ (IC50 = 14 nM)
|
|---|---|
| 体外研究 (In Vitro) |
视黄酸/retinoic acid,也称为全反式视黄酸,或 ATRA,是维生素 A 的高效衍生物,几乎是所有重要的生理过程和功能所必需的。它在 530 多个不同基因的转录控制中发挥作用。视黄酸的作用机制是通过其作为核视黄酸受体 (RARα-γ) 的激活配体的作用,与视黄酸 X 受体 (RXRα-γ) 结合形成异二聚体[1]。视黄酸 (RA) 的 Kd 值在 100 至 200 nM 之间,以低亲和力与 PPARα 和 PPARγ 结合。另一方面,视黄酸与 PPARβ/δ 结合时表现出高亲和力和同种型选择性,Kd 为 17 nM [2]。视黄酸 (RA) 受体 RARα、RARβ、RARγ 和 PPARβ/δ 以及视黄酸结合蛋白 CRABP-II 和 FABP5 由未分化的 P19 细胞表达。用视黄酸处理细胞诱导分化导致 CRABP-II 短暂过度表达和 FABP5 下调,这在相关蛋白质和 mRNA 水平上检测到。经过最初的下降后,成熟神经元中的 FABP5 蛋白和 mRNA 水平与未分化的 P19 细胞相比上升了 2-2.5 倍。 PPARβ/δ和RARα的水平没有受到分化诱导的显着影响。到第 4 天,RARγ mRNA 水平下降了近 5 倍,并且在成熟神经元中保持较低水平 [3]。视黄酸 (RA) 是由视黄醇(维生素 A)产生的一种形态发生素,在细胞发育、分化和器官发生中发挥着至关重要的作用。视黄酸与视黄酸受体 (RAR) 和视黄酸 X 受体 (RXR) 相互作用,调节靶基因的表达 [4]。
UAB30是一种RXR选择性激动剂,已被证明具有潜在的癌症化学预防特性。由于其疗效高、毒性低,目前正由国家癌症研究所在人体I期临床试验中进行评估。虽然UAB30显示出作为低毒化学预防药物的前景,但其作用机制尚不清楚。在这项研究中,我们研究了UAB30对人类器官型皮筏培养物和小鼠表皮基因表达的影响。这项研究的结果表明,用UAB30治疗会导致负责全反式视黄醇摄取和代谢为全反式-视黄酸/retinoic acid(ATRA)的基因上调,ATRA是RAR核受体的天然激动剂。与这些基因表达的增加一致,ATRA在人类皮肤筏中的稳态水平升高。在紫外线B(UVB)照射的小鼠皮肤中,发现ATRA靶基因的表达减少。在UVB诱导的鳞状细胞癌和基底细胞癌小鼠模型的表皮中也观察到ATRA敏感基因的表达减少。然而,在UVB照射前用UAB30治疗小鼠皮肤可以防止UVB诱导的一些ATRA反应基因表达的降低。考虑到UAB30对表皮中的ATRA信号的积极作用及其低毒性,它可以作为一种化学预防剂用于治疗非毛细胞瘤皮肤癌症,特别是在器官移植受者和其他高危人群中。[1] 维甲酸/retinoic acid(RA)调节许多靶基因的转录,从而调节无数的生物过程。众所周知,RA通过激活视黄酸受体(RAR)发挥作用,RAR反过来控制细胞分化、增殖和凋亡。然而,已经发表了关于RA各种不同且有时相反的行为的令人困惑的报道。因此,虽然RA在某些情况下诱导细胞凋亡并抑制细胞生长,但在其他情况下,它会增强增殖并充当抗凋亡剂。这些观察结果提出了除RAR之外的信号通路可能参与调节RA活动的可能性。在这里,我们表明RA是另一种核受体的高亲和力配体,即孤儿受体过氧化物酶体增殖物激活受体(PPAR)β/δ。我们证明,虽然RA不激活PPARα和PPARγ,但它以纳摩尔亲和力与PPARβ/δ结合,调节受体的构象,促进与辅激活子SRC-1的相互作用,并有效激活PPARβ/δ介导的转录。因此,RA的转录信号传导是通过双重途径发挥的,为理解对这种激素的不同细胞反应提供了理论基础。[2] 维甲酸/retinoic acid(RA)通过激活核受体维甲酸受体(RAR)和过氧化物酶体增殖物激活受体(PPAR)β/δ及其各自的同源脂质结合蛋白CRABP-II和FABP5来调节基因转录。RA诱导神经元分化,但激素的两种转录途径对这一过程的贡献尚不清楚。在这里,我们表明RA诱导的P19干细胞向神经元祖细胞的承诺是由CRABP-II/RAR通路介导的,FABP5/PPARβ/δ通路可以通过诱导RAR阻遏物SIRT1和Ajuba来抑制这一过程。与神经发生早期阶段的抑制活性相反,FABP5/PPARβ/δ通路促进神经元祖细胞向成熟神经元的分化,这一活性部分由PPARβ/δ靶基因PDK1介导。因此,RA诱导的神经元分化在早期通过RAR介导,在晚期通过PPARβ/δ介导。RA信号传导的转换是通过RARβ的瞬时上调来实现的,同时伴随着分化早期CRABP-II/FABP5比值的瞬时增加。根据这些结论,与野生型动物相比,FABP5缺失小鼠的海马体显示出神经元祖细胞的过度积累和成熟神经元的缺陷。[3] 视黄酸(RA)是一种来源于视黄醇(维生素a)的形态发生因子,在细胞生长、分化和器官发生中起着重要作用。从视黄醇生产RA需要两组不同脱氢酶催化的连续酶促反应。视黄醇首先被氧化成视网膜,然后被氧化成RA。RA与视黄酸受体(RAR)和视黄酸X受体(RXR)相互作用,然后调节靶基因表达。在这篇综述中,我们讨论了RA的代谢和RA信号通路的重要组成部分,并强调了目前对RA在早期胚胎发育中功能的理解。[4] 维甲酸/retinoic acid(RA)通过激活RA受体(RAR)这一转录因子家族,对细胞生长和分化产生多效作用。这些受体存在三种亚型,RARα、RARβ和RARγ。受体在不同的细胞类型和发育阶段有不同的表达,这表明它们可能调节不同的基因集。我们已经鉴定出一种具有选择性RARα拮抗剂特征的合成维甲酸。这种拮抗剂可以抵消RA对HL-60细胞分化和B淋巴细胞多克隆活化的影响。除了其潜在的实际相关性外,这种和其他特定的拮抗剂将有助于剖析RAR系统,并将许多RA调节的功能分配给一个给定的受体。[5] 异硫氰酸盐和酚类抗氧化剂可以通过激活Nrf2(NF-E2 p45-相关因子2)来预防癌症,Nrf2是一种通过抗氧化反应元件(ARE)增强子控制细胞保护基因表达的转录因子。使用人乳腺MCF7-衍生的AREc32报告细胞系,我们现在报告全反式视黄酸(ATRA)和其他视黄酸受体α(RARAalpha)激动剂显著降低Nrf2介导癌症化学预防剂诱导ARE驱动基因的能力,所述化学预防剂包括丁基羟基茴香醚的代谢产物,叔丁基氢醌(tBHQ)。在AREc32细胞中,由Nrf2调节的醛酮还原酶(AKR)AKR1C1和AKR1C2基因的基础和tBHQ诱导表达也受到ATRA的抑制。RARalpha的拮抗剂增强了tBHQ对ARE驱动基因表达的诱导,使用RNAi敲除RARalpha也是如此[6]。 |
| 体内研究 (In Vivo) |
将浓度为 0.3 μM 的视黄酸 (GMP) 应用于浸入含视黄酸的鱼缸水中的胚胎后,斑马鱼在 24 和 48 小时后表现出更快的视杆细胞分化[6]。
RA抑制小鼠小肠中ARE基因库的表达。[6] 为了研究视黄酸/RA是否在体内抑制ARE调节基因的表达,将Nrf2-/-和Nrf2+/+小鼠置于维生素a缺乏(VAD)饮食中。已知通过Nrf2调节的蛋白质的蛋白质印迹显示,VAD饮食的WT小鼠小肠中Gstm5、GCLC、NQO1和Gsta1/2的水平显著增加(图6)。在VAD饮食的Nrf2−/-小鼠中,这些蛋白质的水平没有增加。对VAD饮食的WT小鼠施用维甲酸/ATRA(10mg/kg,2周腹腔注射)几乎完全阻断了小肠中Gstm5、GCLC、NQO1和Gsta1/2蛋白的增加(图6,泳道5),表明维甲酸在体内抑制Nrf2功能。对对照饮食的WT小鼠施用ATRA不影响Gstm5、GCLC、NQO1或Gsta1/2的表达(数据未显示)。 |
| 酶活实验 |
荧光滴定法[2]
细菌表达的mPPARα-LBD、mPPARβ/δ-LBD和mPPARγ-LBD(0.2-1μm)直接在试管中用溶于乙醇的视黄酸/RA滴定。乙醇浓度通常低于1%,从不超过2%。为了确保蛋白质和配体之间的平衡,监测荧光,直到达到恒定值。通过跟踪伴随RA结合的蛋白质固有荧光(激发,280 nm;发射,340 nm)的降低来监测滴定的进展。配体的内部过滤,由蛋白质饱和后观察到的线性斜率反映,如所述进行了校正。对校正后的数据进行分析,以获得平衡离解常数(Kd)。通过将数据拟合到从简单结合理论导出的方程(1)中进行分析, (方程式1) 其中F是观察到的荧光,F 0和F∞分别是无配体和饱和时的荧光,P T和R T分别是蛋白质和RA/视黄酸的总浓度,Ka是缔合常数(Ka=1/Kd)。 |
| 细胞实验 |
皮肤Raft中维甲酸/ATRA的检测[1]
将UAB30在DMSO(50 mM)中的浓缩溶液的等分试样加入培养基中,使最终浓度达到2μM。每隔一天更换一次培养基,并补充新鲜的UAB30。对照样品的培养基仅补充DMSO。收获后,将筏培养物的表皮从下面的胶原蛋白床上剥离。将UAB30处理或DMSO处理的培养物合并到三个样品中,每个样品包含五个筏,并基本上按照[52]中所述提取维甲酸。在黑暗中,将每个样品在0.5mL冰冷的磷酸盐缓冲盐水(PBS)中均质化,转移到硅化玻璃管中,并与0.5mL含有0.025M氢氧化钾的乙醇混合。用2mL己烷提取非极性维甲酸,在氮气流下干燥有机相,在50μL己烷:乙腈(70:30)中复溶,并如前所述通过反相HPLC进行分析。通过加入45μL 4M盐酸酸化剩余的水相,并用另外2mL己烷提取极性维甲酸(包括ATRA和UAB30)。将提取物干燥并在400μL乙腈中复溶。 组织样本中视黄酸/ATRA的LC-MS-MS分析基本上如前所述进行,但有一些修改。为了量化干燥提取物中存在的ATRA或UAB30的浓度,将50-μL等分试样注入岛津LC-10AD HPLC(包括脱气器),该HPLC与Applied Biosystems 4000 Q Trap质谱仪连接。用于所有分析的柱是SUPELCOSIL ABZ PLUS(10 cm x 2.1 mm,3μm)。使用梯度程序将含有40%乙腈、30%甲醇和30%超纯水的流动相A与含有55%乙腈、30%甲烷和15%超纯水的移动相B混合。每个流动相含有0.01%v/v的甲酸。用于混合流动相的梯度程序为:0至5分钟,100%A至100%B;5至19分钟,100%B;19至20分钟,100%B至100%A;20至30分钟,100%A.流动相的流速保持恒定在200μL/min。质谱仪在Analyst 1.4.2软件控制的多反应监测中使用大气压化学电离(APCI)源运行。ATRA和UAB30的停留时间为40ms。最佳阳性APCI检测条件为:幕气10、喷雾器气3、碰撞气6、离子源70和温度350°C。 每个样品注射三次,三次注射的平均值用于估算其视黄酸/ATRA或UAB30浓度。对于ATRA,四极杆1(Q1)中选择了301 m/z,四极杆3(Q3)中定量了123 m/z的离子碎片离子。对于UAB30,第一季度选择295 m/z,第三季度选择165 m/z。在分析之前,使用Analyst中的优化子程序对每个峰的去簇潜力、入口潜力、碰撞能量和碰撞单元出口潜力进行了优化。 为了定量ATRA水平,使用每种浓度3次注射的0.0-1.6 pmol/50μL视黄酸/ATRA注射液(7种浓度,变化2倍)进行校准曲线。将123m/z峰的总离子电流面积(TIC)拟合为线性方程,以建立校准曲线。将123m/z峰的TIC面积测量三次并取平均值。使用平均峰面积和线性校准曲线测定样品中ATRA的内源性浓度。除了校准曲线和定量使用165 m/z片段峰,以及在构建校准曲线时使用不同浓度范围(0.0-1.0 pmol/50μL UAB30注射液)外,UAB30使用了相同的方法。 Transient Transfection。[6] 使用Lipofectamine 2000试剂(Life Technologies)在70-80%融合率下用Nrf2表达载体转染AREc32细胞。转染后5小时,在有或没有1μM维甲酸/ATRA的情况下,用含有10μM tBHQ的新鲜DMEM替换培养基。对于对照实验,向培养基中加入模拟转染(无质粒DNA)和单独的载体(0.1%体积/体积DMSO)。在收获和分析细胞之前,将细胞放置24小时以对外源性物质产生反应。在对照实验中,将不含DNA的转染试剂单独加入细胞中,用DMSO处理2小时。 对于AREc32细胞中的RAR敲除实验,从Ambion购买了靶向RARαmRNA不同区域的两个预连接siRNA序列1(5′-GGAAUUGUGCUGUUAUUtt-3′)和2(5′GCUCACAUCAUCUCAUCATT-3′)。类似地,使用特异性靶向人RARγ的预先验证的siRNA(5′-GGAAGUGUGCGAAAUGACtt-′)转染AREc32细胞。在这些情况下,siRNA(每孔200 pmol)和Lipofectamine 2000试剂(每孔10μl)在六孔板中用1 ml Optimum稀释,并在20°C下孵育20分钟。此后,4×105个细胞在4 ml不含抗生素的生长培养基中稀释,并直接分配到每个孔中。孵育24小时后,用10μM tBHQ、1μM视黄酸/ATRA或10μM tBHQ加1μM ATRA在新鲜DMEM中处理细胞24小时。 |
| 动物实验 |
Homozygous Nrf2 KO mice were used. Two-month-old C57BL/6 Nrf2−/− and Nrf2+/+ male mice were used in this study. All animal procedures were carried out under a United Kingdom Home Office license and with local ethical approval.
Nrf2−/− and Nrf2+/+ (n = 2–3) mice were placed on a VAD (Special Diet Service) or control diet for 6 weeks and then killed. Nrf2+/+ mice were also placed on a VAD diet for 6 weeks; during the last two weeks, they received either no treatment, retinoic acid/ATRA i.p. daily at 10 mg/kg, or the equivalent volume of corn oil. Mice were killed and the small intestine excised, washed, and frozen in liquid nitrogen. [6]
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Tretinoin applied topically is expected to remain on the stratum corneum and undergo minimal systemic absorption. In one study, the topical application of radiolabelled tretinoin for 28 days was associated with a total percutaneous absorption of 2%. The extent of absorption was examined after a once-daily application of 1.9 g of the combination product with [benzoyl peroxide] for 14 days. On Day 14, at steady-state, the mean Cmax was 0.15-0.19 ng/mL for tretinoin, 0.27-0.34 ng/mL for the metabolite 4-keto 13-cis retinoic acid, and 0.13-0.28 ng/mL for 13-cis retinoic acid, respectively. The Cmax varied across different age groups (children, adolescents, and adults). The corresponding ranges for the mean AUC0-24 were 0.63-2.06, 2.39-2.89, and 0.96-1.99 ng\*h/mL. Following oral administration, the absolute bioavailability of tretinoin was approximately 50%. While the effect of food on tretinoin is unclear, food increases the oral absorption of retinoids, as a class. When the oral dose of 22.5 mg/m2 tretinoin was administered twice daily, the mean ± SD Cmax was 394 ± 89 ng/mL after the first dose and 138 ± 139 ng/mL after one week of continuous treatment. The area under the curve (AUC) was 537 ± 191 ng·h/mL after the first dose and 249 ± 185 ng·h/mL after one week of continuous treatment. The Tmax was between one and two hours. Tretinoin metabolites are excreted in bile and urine. Following administration of radiolabeled tretinoin at doses of 2.75 mg and 50 mg - which are 0.53 to 9.6 times the approved recommended dosage based on 1.7 m2, respectively - approximately 63% of the radioactivity was recovered in the urine within 72 hours, and 31% appeared in the feces within six days. Tretinoin is rapidly and extensively distributed to tissues following oral administration but does not cross the blood-brain barrier. The apparent volume of distribution (Vd) of intravenous tretinoin is dose-dependent and significantly greater at low doses. The Vd was 0.52 ± 0.12 L/kg after 0.0125 mg/kg and 0.21 ± 0.05 L/kg after 0.25 mg/kg. No information is available. /MILK/ It is not known whether topically applied tretinoin is excreted in human milk. Studies with radiolabeled drug have demonstrated that after the oral administration of 2.75 and 50 mg doses of tretinoin, greater than 90% of the radioactivity was recovered in the urine and feces. Based upon data from 3 subjects, approximately 63% of radioactivity was recovered in the urine within 72 hours and 31% appeared in the feces within 6 days. A single 45 mg/sq m (approximately 80 mg) oral dose to APL /acute promyelocytic leukemia/ patients resulted in a mean +/- SD peak tretinoin concentration of 347 +/- 266 ng/mL. Time to reach peak concentration was between 1 and 2 hours. The apparent volume of distribution of tretinoin has not been determined. Tretinoin is greater than 95% bound in plasma, predominately to albumin. Plasma protein binding remains constant over the concentration range of 10 to 500 ng/mL. For more Absorption, Distribution and Excretion (Complete) data for all-trans-Retinoic acid (12 total), please visit the HSDB record page. Metabolism / Metabolites Tretinoin is rapidly metabolized to form various oxidized and conjugated metabolites. It forms several metabolites stereoisomerization derivatives (9-_cis_-retinoic acid or [alitretinoin] and 13-_cis_-retinoic acid or [isotretinoin]), oxidation derivatives (4-hydroxy-retinoic acid, 4-oxo-retinoic acid, 18-hydroxy-retinoic acid, 5,6-epoxy-retinoic acid, 3,4-didehydro-retinoic acid and retinotaurine), stereoisomerization and oxidation derivatives (13-_cis_-4-oxo-retinoic acid), glucuronidation derivatives (retinoyl beta-glucuronide, 13-_cis_-retinoyl beta-glucuronide, 4-oxo-retinoyl beta-glucuronide, 5,6-epoxyretinoyl beta-glucuronide and 13-_cis_-4-oxo-retinoyl beta-glucuronide), nonpolar metabolites of retinoic acid, and retinoic acid esters. Tretinoin is metabolized by several CYP enzymes, including CYP3A4, CYP2C8, and CYP2E. It also undergoes glucuronidation by UGT2B7. The metabolites 4-oxo retinoic acid and 4-oxo _trans_ retinoic acid glucuronide have one-third of the pharmacological activity of the parent compound. When the plasma concentrations decreased to one-third of their day-one concentrations after one week of continuous therapy, tretinoin induced its own metabolism. Evidence suggests that tretinoin induces its own metabolism. In patients with APL receiving 45 mg/sq m tretinoin daily, urinary excretion of 4-oxo trans retinoic acid glucuronide increased approximately tenfold over the course of 2-6 weeks of continuous therapy, suggesting that increased metabolism of tretinoin may be the primary mechanism leading to the decreased plasma drug concentrations observed during continued administration. Possible mechanisms for the increased clearance of tretinoin with continuous daily dosing of the drug include induction of CYP enzymes or oxidative cofactors and increased expression of cellular retinoic acid binding proteins. Increasing the dosage of tretinoin to compensate for the apparent autoinduction has not been shown to increase therapeutic response. Reduced plasma retinoid concentrations have been associated with relapse and clinical resistance, and some investigators suggest that the clinical failure of tretinoin may be related to a lack of sustained effective concentrations of the drug during prolonged treatment. Tretinoin metabolites have been identified in plasma and urine. Cytochrome P450 enzymes have been implicated in the oxidative metabolism of tretinoin. Metabolites include 13- cis retinoic acid, 4-oxo trans retinoic acid, 4-oxo cis retinoic acid, and 4-oxo trans retinoic acid glucuronide. In APL /acute promyelocytic leukemia/ patients, daily administration of a 45 mg/SQ m dose of tretinoin resulted in an approximately tenfold increase in the urinary excretion of 4-oxo trans retinoic acid glucuronide after 2 to 6 weeks of continuous dosing, when compared to baseline values. Ethanol fed rats showed enhanced microsomal retinoic acid metabolism (50%) accompanied by increased microsomal cytochrome P450 content (34%). The increased hepatic microsomal cytochrome P450 dependent metabolism of retinoic acid after chronic ethanol consumption may contribute to the accelerated catabolism of retinoic acid in vivo. Following ip administration of high doses of 15-(14)C- and 10,11-(3)H-labeled retinoic acid to rats, 3 major metabolites were isolated from feces in microgram amounts by column, thin-layer and high-pressure liquid chromatography. Mass spectrometry provided identification as all-trans-4-oxoretinoic acid, all-trans-5'-hydroxy-retinoic acid and 7-trans-9-cis-11-trans-13-trans-5'-hydroxyretinoic acid. For more Metabolism/Metabolites (Complete) data for all-trans-Retinoic acid (8 total), please visit the HSDB record page. Tretinoin has known human metabolites that include 5,6-Epoxy-retinoic acid, All-trans-retinoyl glucuronide, 18-Hydroxyretinoic acid, and 4-Hydroxyretinoic acid. Tretinoin is a known human metabolite of retinal. Hepatic Half Life: 0.5-2 hours Biological Half-Life The terminal elimination half-life of tretinoin following initial dosing is 0.5 to 2 hours in patients with APL. In patients with APL /acute promyelocytic leukemia/ receiving tretinoin orally, a terminal elimination half-life of 0.5-2 hours has been reported following initial dosing. Metabolism of vitamin A and the production of all-trans retinoic acid [4] Vitamin A is a necessary dietary vitamin for the normal development and vision. The critical necessity of vitamin A was hinted as early as 1881 by Nikolai Lunin, who discovered that purified protein, fat, and carbohydrate did not sustain the normal growth of mice, unless the diet was supplemented with milk. Elmer Verner McCollum, then determined in 1917 that the critical component concerned in milk was actually a "fat-soluble factor A", named in contrast to the previously discovered "water-soluble factor B", or vitamin B. These discoveries allowed Carl Edvard Bloch, a Denmark paediatrician, to identify vitamin A deficiency as the cause of night blindness, or xerophthalmia. While vitamin A was a necessary dietary vitamin, vitamin A itself is not the main bioactive mediator of its function. The key mediators of vitamin A function were identified as atRA and 11-cis retinal. atRA is a regulator of gene transcription, while 11-cis retinal acts as a chromophore for visual functions. In this section, we will review the metabolic processes of converting vitamin A into various retinoids, with emphasis on the production of atRA (Figure 1). |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: All-trans-Retinoic acid (tretinoin) indicated for topical application in the treatment of acne vulgari. Tretinoin capsules are indicated for the induction of remission in patients with acute promyelocytic leukemia. HUMAN STUDIES: Cardiac failure occurred in 6% of patients receiving tretinoin, and cardiac arrest, myocardial infarction, stroke, and pulmonary hypertension each occurred in 3% of patients. There is a risk of arterial or venous thrombosis, involving any organ system, during the first month of tretinoin therapy. Thromboembolic events, including fatal pulmonary embolism, have been reported in patients receiving tretinoin. In one patient receiving tretinoin, fatal thromboembolism occurred during concomitant therapy with an antifibrinolytic agent. Bone marrow necrosis, sometimes fatal, has been reported in several patients receiving hydroxyurea during tretinoin therapy. Thrombocytosis has been reported rarely in patients receiving tretinoin. Rapidly evolving leukocytosis occurs in approximately 40% of patients receiving tretinoin. Retinoic acid-acute promyelocytic leukemia (RA-APL) syndrome (also known as APL differentiation syndrome), characterized by fever, dyspnea, acute respiratory distress, weight gain, pulmonary infiltrates, pleural and pericardial effusions, edema, hepatic failure, renal failure, and multiorgan failure, occurs in approximately 25% of patients receiving tretinoin for the treatment of APL. RA-APL syndrome occasionally has been accompanied by impaired myocardial contractility and episodic hypotension and can occur with or without concomitant leukocytosis. In severe cases, progressive hypoxemia requiring endotracheal intubation and mechanical ventilation may occur, and deaths secondary to progressive hypoxemia and multiorgan failure have been reported. ANIMAL STUDIES: There was no evidence of carcinogenic potential when tretinoin dosages of 0.025 mg/kg daily were administered topically to mice. When mice received 0.017 and 0.035% formulations of tretinoin applied topically, cutaneous squamous cell carcinomas and papillomas in the treatment area were observed in some female mice and dose-related hepatic tumors were observed in male mice. Experiments in vitro with cultured rat conceptuses have shown that tretinoin is a direct-acting dysmorphogen. Major defects involved the branchial arches and somites. Retinoid-induced malformations of the jaw, ears, face, skull, eyes, and heart in humans and rodents are well known. In mice that were administered a single oral dose of 100 mg/kg tretinoin on gestation days 9 or 11 and were killed on gestation day 17 skeletal defects (limbs) and cleft palate were present in 90% of the fetuses. There is evidence for teratogenicity (shortened or kinked tail) of topical tretinoin in rats at dosages exceeding 1 mg/kg daily. Bone anomalies also have been reported in rats when tretinoin 10 mg/kg daily was applied dermally. Topical tretinoin cream was associated with an increased incidence of cleft palate and hydrocephaly in rabbits. In rabbits treated with topical tretinoin an increased incidence of domed head and hydrocephaly was noted in some of the fetuses, typical of retinoid-induced fetal malformations in this species. Gross external, soft tissue and skeletal alterations occurred at doses higher than 0.7 mg/kg/day in mice, 2 mg/kg/day in rats, 7 mg/kg/day in hamsters, and at a dose of 10 mg/kg/day, the only dose tested, in pigtail monkey. When given subcutaneously to rabbits, tretinoin was teratogenic at a dosage of 2 mg/kg daily but not at 1 mg/kg daily. In vivo and in vitro (Ames) tests have not demonstrated that tretinoin is mutagenic. However, ingredients in the microsphere formulation of the drug have shown potential for genetic toxicity and teratogenesis. ECOTOXICITY STUDIES: In Japanese flounder, Paralichthys olivaceus, at 6-9 days post-hatching tretinoin induced the most severe deformity in all skeletons examined among retinoic acid isomers. Tretinoin binds to alpha, beta, and gamma retinoic acid receptors (RARs). RAR-alpha and RAR-beta have been associated with the development of acute promyelocytic leukemia and squamous cell cancers, respectively. RAR-gamma is associated with retinoid effects on mucocutaneous tissues and bone. Although the exact mechanism of action of tretinoin is unknown, current evidence suggests that the effectiveness of tretinoin in acne is due primarily to its ability to modify abnormal follicular keratinization. Comedones form in follicles with an excess of keratinized epithelial cells. Tretinoin promotes detachment of cornified cells and the enhanced shedding of corneocytes from the follicle. By increasing the mitotic activity of follicular epithelia, tretinoin also increases the turnover rate of thin, loosely-adherent corneocytes. Through these actions, the comedo contents are extruded and the formation of the microcomedo, the precursor lesion of acne vulgaris, is reduced. Tretinoin is not a cytolytic agent but instead induces cytodifferentiation and decreased proliferation of APL cells in culture and in vivo. When Tretinoin is given systemically to APL patients, tretinoin treatment produces an initial maturation of the primitive promyelocytes derived from the leukemic clone, followed by a repopulation of the bone marrow and peripheral blood by normal, polyclonal hematopoietic cells in patients achieving complete remission (CR). The exact mechanism of action of tretinoin in APL is unknown. Interactions Mouse mammary gland organ culture technique was utilized to determine the effects of retinoids, including trans-retinoic acid, on the prolactin-induced structural differentiation of the mammary gland. Thoracic glands from BALB/C mice pretreated with steroids differentiate in 6 days into alveolar structures in presence of insulin and prolactin. Trans-retinoic acid inhibited prolactin-induced structural changes in the glands. To determine whether 2,3,7,8-tetrachlorodibenzo-p-dioxin and retinoic acid would enhance or antagonize the teratogenic effects of the other compound, C57BL/6N dams were treated orally on gestation days 10 or 12 with 10 ml corn oil/kg containing 2,3,7,8-tetrachlorodibenzo-p-dioxin (0-18 ug/kg), retinoic acid (0-200 mg/kg), or combinations of the two chemicals. Dams were killed on gestation day 18 and toxicity and teratogenicity assessed. Coadministration of 2,3,7,8-tetrachlorodibenzo-p-dioxin and retinoic acid had no effect on maternal or fetal toxicity beyond what would be expected by either compound alone. Cleft palate was induced by retinoic acid at lower doses on gestation day 10 than on gestation day 12, but by 2,3,7,8-tetrachlorodibenzo-p-dioxin at lower doses on gestation day 12 than on gestation day 10. Sensitivity to 2,3,7,8-tetrachlorodibenzo-p-dioxin induced hydronephrosis was similar on both gestation days 10 and 12. The limb bud defects were only observed when retinoic acid was administered on gestation day 10, not when given on gestation day 12. No other soft tissue or skeletal malformations were related to administration of 2,3,7,8-tetrachlorodibenzo-p-dioxin or retinoic acid. No effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin was observed on the incidence or severity of limb bud defects induced by retinoic acid, nor did retinoic acid influence the incidence or severity of hydronephrosis induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. However, the incidence of cleft palate was dramatically enhanced by coadministration of the xenobiotic and vitamin. On both gestation day 10 and 12, the dose-response curves for cleft palate induction were parallel, suggesting some similarities in mechanism between the two compounds. However, combination treatment resulted in a synergistic response that varied with the stage of development and was tissue specific. Risk of pseudotumor cerebri (intracranial hypertension) is increased in patients receiving tretinoin. Concomitant use of other agents known to cause pseudotumor cerebri or intracranial hypertension, such as tetracyclines, may increase the risk of this condition in patients receiving tretinoin. Concurrent use of hydroxyurea, which is cytotoxic to cells in S phase, and tretinoin, which induces cells to enter the S phase, may cause a synergistic effect leading to massive cell lysis. Bone marrow necrosis, sometimes fatal, has been reported in patients receiving hydroxyurea during tretinoin therapy. Although some clinicians have administered hydroxyurea in conjunction with tretinoin therapy to reduce leukocytosis, the safety and efficacy of this practice have not been established, and caution is recommended in the use of hydroxyurea in patients receiving tretinoin. For more Interactions (Complete) data for all-trans-Retinoic acid (14 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral 1960 mg/kg LD50 Rat ip 96 mg/kg LD50 Rat sc 53 mg/kg LD50 Rat iv 78 mg/kg For more Non-Human Toxicity Values (Complete) data for all-trans-Retinoic acid (12 total), please visit the HSDB record page. |
| 参考文献 |
[1]. Retinoid X Receptor Agonists Upregulate Genes Responsible for the Biosynthesis of All-Trans-Retinoic Acid in Human Epidermis. PLoS One. 2016 Apr 14;11(4):e0153556.
[2]. Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor beta/delta. J Biol Chem. 2003 Oct 24;278(43):41589-92. [3]. Retinoic acid induces neurogenesis by activating both retinoic acid receptors (RARs) and peroxisomeproliferator-activated receptor β/δ (PPARβ/δ). J Biol Chem. 2012 Dec 7;287(50):42195-205. [4]. Retinoic acid synthesis and functions in early embryonic development. Cell Biosci. 2012 Mar 22;2(1):11. [5]. A retinoic acid receptor alpha antagonist selectively counteracts retinoic acid effects. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7129-33. [6]. Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alpha. Proc Natl Acad Sci U S A. 2007 Dec 4;104(49):19589-94 |
| 其他信息 |
Therapeutic Uses
Antineoplastic Agents Keratolytic Agents /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. trans-Retinoic acid is included in the database. Tretinoin gel and cream are indicated for topical application in the treatment of acne vulgaris. The safety and efficacy of the long-term use of this product in the treatment of other disorders have not been established. /Included in US product labeling; Tretinoin, topical/ Tretinoin is used topically as a 0.05 or 0.1% cream for palliative therapy to improve dermatologic changes (e.g., fine wrinkling, mottled hyperpigmentation, roughness) associated with photodamage. /NOT included in US product labeling; Tretinoin, topical/ For more Therapeutic Uses (Complete) data for all-trans-Retinoic acid (9 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ Experienced Physician and Institution. Patients with acute promyelocytic leukemia (APL) are at high risk in general and can have severe adverse reactions to tretinoin capsules. Tretinoin capsules should therefore be administered only to patients with APL under the strict supervision of a physician who is experienced in the management of patients with acute leukemia and in a facility with laboratory and supportive services sufficient to monitor drug tolerance and protect and maintain a patient compromised by drug toxicity, including respiratory compromise. Use of tretinoin capsules requires that the physician concludes that the possible benefit to the patient outweighs the following known adverse effects of the therapy. /Tretinoin, systemic/ /BOXED WARNING/ Retinoic Acid-APL Syndrome. About 25% of patients with APL treated with tretinoin capsules have experienced a syndrome called the retinoic acid-APL (RA-APL) syndrome characterized by fever, dyspnea, acute respiratory distress, weight gain, radiographic pulmonary infiltrates, pleural and pericardial effusions, edema, and hepatic, renal, and multi-organ failure. This syndrome has occasionally been accompanied by impaired myocardial contractility and episodic hypotension. It has been observed with or without concomitant leukocytosis. Endotracheal intubation and mechanical ventilation have been required in some cases due to progressive hypoxemia, and several patients have expired with multi-organ failure. The syndrome generally occurs during the first month of treatment, with some cases reported following the first dose of tretinoin capsules. The management of the syndrome has not been defined rigorously, but high-dose steroids given at the first suspicion of the RA-APL syndrome appear to reduce morbidity and mortality. At the first signs suggestive of the syndrome (unexplained fever, dyspnea and/or weight gain, abnormal chest auscultatory findings or radiographic abnormalities), high-dose steroids (dexamethasone 10 mg intravenously administered every 12 hours for 3 days or until the resolution of symptoms) should be immediately initiated, irrespective of the leukocyte count. The majority of patients do not require termination of tretinoin capsules therapy during treatment of the RA-APL syndrome. However, in cases of moderate and severe RA-APL syndrome, temporary interruption of tretinoin capsules therapy should be considered. /Tretinoin, systemic/ /BOXED WARNING/ During tretinoin capsules treatment about 40% of patients will develop rapidly evolving leukocytosis. Patients who present with high WBC at diagnosis (>5x10 9/L) have an increased risk of a further rapid increase in WBC counts. Rapidly evolving leukocytosis is associated with a higher risk of life-threatening complications. If signs and symptoms of the RA-APL syndrome are present together with leukocytosis, treatment with high-dose steroids should be initiated immediately. Some investigators routinely add chemotherapy to tretinoin capsules treatment in the case of patients presenting with a WBC count of >5x10 9/L or in the case of a rapid increase in WBC count for patients leukopenic at start of treatment, and have reported a lower incidence of the RA-APL syndrome. Consideration could be given to adding full-dose chemotherapy (including an anthracycline if not contraindicated) to the tretinoin capsules therapy on day 1 or 2 for patients presenting with a WBC count of >5x10 9/L, or immediately, for patients presenting with a WBC count of <5x10 9/L, if the WBC count reaches >/= 6x10(9)/L by day 5, or >/= 10x10(9)/L by day 10, or >/=15x10(9)/L by day 28. /Tretinoin, systemic/ /BOXED WARNING/ Teratogenic Effects. Pregnancy Category D. There is a high risk that a severely deformed infant will result if tretinoin capsules are administered during pregnancy. If, nonetheless, it is determined that tretinoin capsules represent the best available treatment for a pregnant woman or a woman of childbearing potential, it must be assured that the patient has received full information and warnings of the risk to the fetus if she were to be pregnant and of the risk of possible contraception failure and has been instructed in the need to use two reliable forms of contraception simultaneously during therapy and for 1 month following discontinuation of therapy, and has acknowledged her understanding of the need for using dual contraception, unless abstinence is the chosen method. Within 1 week prior to the institution of tretinoin capsules therapy, the patient should have blood or urine collected for a serum or urine pregnancy test with a sensitivity of at least 50 mIU/mL. When possible, tretinoin capsules therapy should be delayed until a negative result from this test is obtained. When a delay is not possible, the patient should be placed on two reliable forms of contraception. Pregnancy testing and contraception counseling should be repeated monthly throughout the period of tretinoin capsules treatment. /Tretinoin, systemic/ For more Drug Warnings (Complete) data for all-trans-Retinoic acid (44 total), please visit the HSDB record page. Pharmacodynamics Tretinoin is a vitamin A derivative that promotes cell production, proliferation, and differentiation. When used topically, tretinoin regulates epidermal cell turnover and collagen production. It also prevents collagen loss, reduces inflammation, and blocks the induction of matrix metalloproteinase (MMP), which are enzymes that disrupt collagen and elastic fibres. In short-term and long-term studies, topical application of tretinoin at doses ranging from 0.001% to 0.1% was associated with improvements in clinical signs of photoaging and fine wrinkles, increased epidermal thickness, compaction of the stratum corneum, and decreased melanin content. It also improved melanocyte differentiation and distribution, promotion of epidermal hyperplasia, and angiogenesis. Tretinoin exhibits antineoplastic activities when given orally. Tretinoin was shown to induce differentiation in tumour cells. It induced cytodifferentiation and decreased acute promyelocytic leukemia (APL) cell proliferation in culture and _in vivo_. In patients with APL, tretinoin promoted the initial maturation of the primitive promyelocytes derived from the leukemic clone, followed by a repopulation of the bone marrow and peripheral blood by normal, polyclonal hematopoietic cells in patients achieving complete remission. |
| 分子式 |
C20H28O2
|
|---|---|
| 分子量 |
300.4
|
| 精确质量 |
300.208
|
| 元素分析 |
C, 79.96; H, 9.39; O, 10.65
|
| CAS号 |
302-79-4
|
| 相关CAS号 |
Retinoic acid-d5;78996-15-3;Retinoic acid;302-79-4;11-cis-Retinoic Acid-d5;Retinoic acid-d6;2483831-72-5
|
| PubChem CID |
444795
|
| 外观&性状 |
Yellow to light-orange crystalline powder
Crystals from ethanol |
| 密度 |
1.0±0.1 g/cm3
|
| 沸点 |
462.8±14.0 °C at 760 mmHg
|
| 熔点 |
179-184ºC
|
| 闪点 |
350.6±11.0 °C
|
| 蒸汽压 |
0.0±2.5 mmHg at 25°C
|
| 折射率 |
1.556
|
| LogP |
6.83
|
| tPSA |
37.3
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
567
|
| 定义原子立体中心数目 |
0
|
| SMILES |
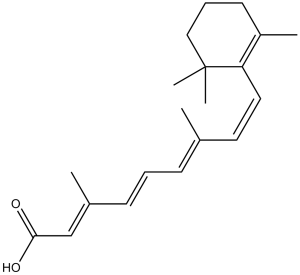
CC1(C)C(/C=C/C(C)=C/C=C/C(C)=C/C(O)=O)=C(C)CCC1
|
| InChi Key |
SHGAZHPCJJPHSC-YCNIQYBTSA-N
|
| InChi Code |
InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+
|
| 化学名 |
(2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenoic acid
|
| 别名 |
All-trans Retinoic Acid; Ro 5488; Ro-5488; tretinoin; ATRA; Renova; Aknefug; Atralin; Retin-A Micro; Tretinoina; ...; 302-79-4; Vitamin A acid; ATRA; TRA; Ro5488; alltrans vitamin A acid; betaretinoic acid; retinoic acid; TRA; trans retinoic acid; trans vitamin A acid; tretinoinum; Trade names: Avita; Renova; Aberel; Aknoten; RetinA; RetinA MICRO; Vesanoid. Foreign brand names: Airol; Eudyna; RetisolA; StievaA; Cordes Vas; Dermairol; EpiAberel; StievaA Forte; Vitinoin
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (8.32 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液; 超声和加热处理
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.32 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (8.32 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 2.5 mg/mL (8.32 mM) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 5 中的溶解度: 2.5 mg/mL (8.32 mM) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 6 中的溶解度: 5 mg/mL (16.64 mM) in 50% PEG300 50% PBS (这些助溶剂从左到右依次添加,逐一添加), 悬浮液; 需要超声助溶并加热至 40°C。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3289 mL | 16.6445 mL | 33.2889 mL | |
| 5 mM | 0.6658 mL | 3.3289 mL | 6.6578 mL | |
| 10 mM | 0.3329 mL | 1.6644 mL | 3.3289 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06358677 | Not yet recruiting NEW | Drug: Larotrectinib Sulfate Procedure: Bone Scan |
Metastatic Colon Cancer Metastatic Rectum Cancer |
NCT06358677 | July 2024 | Phase 2 |
| NCT04724473 | Completed | Drug: Tretinoin Cream 0.025% | Acne Vulgaris | Taro Pharmaceuticals USA | December 10, 2019 | Early Phase 1 |
| NCT03200847 | Active, not recruiting Has Results | Drug: Pembrolizumab with All-Trans Retinoic Acid |
Stage IV Melanoma Stage III Melanoma |
University of Colorado, Denver | October 31, 2017 | Phase 1 Phase 2 |
| NCT06213987 | Recruiting | Drug: 0.025% Tretinoin | Acanthosis Nigricans Hyperpigmentation |
Srinakharinwirot University | February 1, 2024 | Phase 3 |