| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 500mg |
|
| 体内研究 (In Vivo) |
已证明L-天冬氨酸是一种适合结肠特异性药物递送的前药[1]。预给予 100 mM L-天冬氨酸和 100 mM L-Glu 可使 5 分钟时大脑中仍然存在的 L-[3H]Asp 量分别增加 206% 和 178%; L-[3H]Asp 外排转运不受 100 mM D-Asp 影响。 100 mM L-天冬氨酸和 100 mM L-Glu 的共同给药分别使 20 分钟时 L-[3H]Glu 的值增加了 145% 和 156%,但 D-Asp 没有增加。值得注意的是,L-天冬氨酸穿过血脑屏障的速度似乎是 L-谷氨酸的七倍[2]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorbed from the small intestine by an active transport process ASPARTIC ACID PLASMA CONCN WAS ELEVATED 30 MIN AFTER 1 G/KG L-ASPARTATE (ORAL OR IP) TO 15 DAY OLD & ADULT MICE. THEREAFTER, CONCN DECLINED EXPONENTIALLY WITH T/2 OF 0.2 HR IN BOTH. PLASMA CONCN NOT APPRECIABLY ALTERED BY 10 & 100 MG/KG L-ASPARTATE ORAL OR IP ADMIN. Following ingestion, L-aspartate is absorbed from the small intestine by an active transport process. Following absorption, L-aspartate enters the portal circulation and from there is transported to the liver, where much of it is metabolized to protein, purines, pyrimidines and L-arginine, and is catabolized as well. L-aspartate is not metabolized in the liver; it enters the systemic circulation, which distributes it to various tissues of the body. The cations associated with L-aspartate independently interact with various substances in the body and participate in various physiological processes. /L-aspartate/ ... Contents of D- and L-aspartic acids in rats at different stages of growth (from 1 day before birth to 90 days after birth) were determined. D-Aspartic acid was detected in all the brain tissue samples tested, but at different levels. In the cerebrum of rats 1 day before birth, D-aspartic acid was found to be at the highest concentration of 81 nmol/g wet tissue. The level of D-aspartic acid in rat brain falls rapidly after birth, while the L-aspartic acid level increases with age. Enzymatic synthesis of 11C-(4)-L-aspartic acid was undertaken using commercially available wheat germ phosphoenolpyruvate carboxylase. Whole-body distribution of the radioactive compound in rats showed higher accumulation in the salivary gland, glandular stomach and the pancreas, as well as in the lungs. Within 60 minutes after intravenous injection of 11C-(4)-L-aspartic acid, about 60% is removed as 11CO2 by expiration, indicating that the carbon atom at the fourth position of the radioactive compound is easily subjected to decarboxylation. The brain efflux index method has been used to clarify the mechanism of efflux transport of acidic amino acids such as L-aspartic acid (L-Asp), L-glutamic acid (L-Glu), and D-aspartic acid (D-Asp) across the blood-brain barrier (BBB). About 85% of L-[3H]Asp and 40% of L-(3H)Glu was eliminated from the ipsilateral cerebrum within, respectively, 10 and 20 min of microinjection into the brain. The efflux rate constant of L-(3H)Asp and L-(3H)Glu was 0.207 and 0.0346 min(-1), respectively. However, D-(3H)Asp was not eliminated from brain over a 20-min period. The efflux of L-(3H)Asp and L-(3H)Glu was inhibited in the presence of excess unlabeled L-Asp and L-Glu, whereas D-Asp did not inhibit either form of efflux transport. Aspartic acid efflux across the BBB appears to be stereospecific. Using a combination of TLC and the bioimaging analysis, attempts were made to detect the metabolites of L-(3H)Asp and L-(3H)Glu in the ipsilateral cerebrum and jugular vein plasma following a microinjection into parietal cortex, area 2. Significant amounts of intact L-(3H)Asp and L-(3H)Glu were found in all samples examined, including jugular vein plasma, providing direct evidence that at least a part of the L-Asp and L-Glu in the brain interstitial fluid is transported across the BBB in the intact form. To compare the transport of acidic amino acids using brain parenchymal cells, brain slice uptake studies were performed. Although the slice-to-medium ratio of D-(3H)Asp was the highest, followed by L-[3H]Glu and L-[3H]Asp, the initial uptake rate did not differ for both L-(3H)Asp and D-(3H)Asp, suggesting that the uptake of aspartic acid in brain parenchymal cells is not stereospecific. These results provide evidence that the BBB may act as an efflux pump for L-Asp and L-Glu to reduce the brain interstitial fluid concentration and act as a static wall for D-Asp. Metabolism / Metabolites FOR L-ASPARTIC ACID, OXALOACETIC ACID IS PRODUCT OF OXIDATIVE DEAMINATION OR TRANSAMINATION; ALPHA-ALANINE IS PRODUCT OF DECARBOXYLATION. /FROM TABLE/ METABOLIC PATHWAYS & PRODUCTS /IN ANIMAL BODY/: ASPARTIC ACID + CARBAMYLPHOSPHATE /PRODUCE/ PHOSPHORUS + CARBAMYLASPARTIC ACID GIVE PYRIMIDINES; ASPARTIC ACID /PRODUCES/ FUMARIC ACID + NH3; ASPARTIC ACID /PRODUCES/ ASPARTIC SEMIALDEHYDE /PRODUCES/ HOMOSERINE /PRODUCES/ (I) THREONINE, (II) METHIONINE, OR (III) LYSINE... /FROM TABLE/ METABOLIC PATHWAYS & PRODUCTS /IN ANIMAL BODY/: ASPARTIC ACID GIVES NITROGEN OF PURINE RING...ASPARTIC ACID + IMP GIVE ADENYLOSUCCINATE GIVES AMP + FUMARATE... /FROM TABLE/ Following ingestion, L-aspartate is absorbed from the small intestine by an active transport process. Following absorption, L-aspartate enters the portal circulation and from there is transported to the liver, where much of it is metabolized to protein, purines, pyrimidines and L-arginine, and is catabolized as well. D-aspartate is not metabolized in the liver; it enters the systemic circulation, which distributes it to various tissues of the body. The cations associated with L-aspartate independently interact with various substances in the body and participate in various physiological processes. /L-aspartate; D-aspartate/ |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
ASPARTIC ACID PREVENTED TO SOME EXTENT THE APPEARANCE OF SYMPTOMS OF PHYSICAL MORPHINE DEPENDENCE IN BALB/C MICE. The effects of amino acids on the embryotoxicity and placental transfer of nickel chloride, day 10 rat embryos were cultured in rat serum medium containing nickel chloride or NiCl2-63 (0.34 or 0.68 uM NiCl with or without L-histidine (2 uM), L-aspartic acid, glycine (2 or 8 uM) or L-cysteine (2 uM). After 26 hr, conceptuses were assessed for survival, growth and development and malformations. The nickel-63 contents of embryos and yolk sacs and the extent of Nickel-63 binding to the proteins of the culture medium were also determined. Nickel chloride alone did not affect the embryonic development at 0.34 uM and caused growth retardation and brain and caudal abnormalities at 0.68 uM. Coincubation of L-histidine, L-cysteine, or L-aspartic acid 0.68 uM Ni reduced the growth retardation and the incidence and/or severity of brain defects caused by nickel chloride and decreased the concentrations of nickel-63 in the yolk sacs compared to 0.68 uM nickel-63 alone. In the presence of L-histidine, L-cysteine or L-aspartic acid there was a shift of nickel-63 binding from the high molecular weight proteins of the culture medium to the low molecular weight fraction. The effect of oral D-aspartic acid and/or L-aspartic acid (aspartic acid) on the body weight of rats was studied. Rats given the D- or D- plus L-isomers showed a greater decrease in weight and in protein, triglyceride and glycogen than did rats given the L-isomer alone. The results were discussed with reference to amino acid antagonism of opioids. |
| 参考文献 |
|
| 其他信息 |
L-aspartic acid is the L-enantiomer of aspartic acid. It has a role as an Escherichia coli metabolite, a mouse metabolite and a neurotransmitter. It is an aspartate family amino acid, a proteinogenic amino acid, an aspartic acid and a L-alpha-amino acid. It is a conjugate acid of a L-aspartate(1-). It is an enantiomer of a D-aspartic acid.
One of the non-essential amino acids commonly occurring in the L-form. It is found in animals and plants, especially in sugar cane and sugar beets. It may be a neurotransmitter. L-Aspartic acid is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Aspartic acid has been reported in Streptomyces akiyoshiensis, Pinus densiflora, and other organisms with data available. Aspartic Acid is a non-essential amino acid in humans, Aspartic Acid has an overall negative charge and plays an important role in the synthesis of other amino acids and in the citric acid and urea cycles. Asparagine, arginine, lysine, methionine, isoleucine, and some nucleotides are synthesized from aspartic acid. Aspartic acid also serves as a neurotransmitter. (NCI04) One of the non-essential amino acids commonly occurring in the L-form. It is found in animals and plants, especially in sugar cane and sugar beets. It may be a neurotransmitter. Drug Indication There is no support for the claim that aspartates are exercise performance enhancers, i.e. ergogenic aids. Mechanism of Action There are also claims that L-aspartate has ergogenic effects, that it enhances performance in both prolonged exercise and short intensive exercise. It is hypothesized that L-aspartate, especially the potassium magnesium aspartate salt, spares stores of muscle glycogen and/or promotes a faster rate of glycogen resynthesis during exercise. It has also been hypothesized that L-aspartate can enhance short intensive exercise by serving as a substrate for energy production in the Krebs cycle and for stimulating the purine nucleotide cycle. Therapeutic Uses MEDICATION (VET): TO REDUCE AMMONIA BLOOD LEVELS & SAID TO BE OF VALUE IN OVERCOMING FATIGUE. ... DOSAGE: GIVEN ORALLY OR AS FEED ADDITIVE AGAINST STRESS INDUCED HIGH BLOOD AMMONIA LEVELS IN POULTRY & AGAINST AMMONIA INTOXICATED RATS. /ASPARTIC ACID/ Parenteral nutrition /EXPL/: L-aspartate is a glycogenic amino acid, and it can also promote energy production via its metabolism in the Krebs cycle. These latter activities were the rationale for the claim that supplemental aspartate has an anti-fatigue effect on skeletal muscle, a claim that was never confirmed. /L-aspartate/ There are claims that L-aspartate is a special type of mineral transporter for cations, such as magnesium, into cells. Magnesium aspartate has not been found to be more biologically effective when compared with other magnesium salts. There are also claims that L-aspartate has ergogenic effects, that it enhances performance in both prolonged exercise and short intensive exercise. It is hypothesized that L-aspartate, especially the potassium magnesium aspartate salt, spares stores of muscle glycogen and/or promotes a faster rate of glycogen resynthesis during exercise. It has also been hypothesized that L-aspartate can enhance short intensive exercise by serving as a substrate for energy production in the Krebs cycle and for stimulating the purine nucleotide cycle. An animal study using injected aspartate failed to find any evidence of a glycogen-sparing effect or any ergogenic effects whatsoever. A more recent double-blind human study of male weight trainers similarly found aspartate supplementation to have no effect, and another study of the effect of aspartate on short intensive exercise again found no effect. /L-aspartate/ For more Therapeutic Uses (Complete) data for (L)-ASPARTIC ACID (6 total), please visit the HSDB record page. Drug Warnings Mild gastrointestinal side effects including diarrhea have been reported. /L-aspartate/ Because of lack of long-term safety studies, L-aspartate salts should be avoided by children, pregnant women and lactating women. /L-Aspartate/ The effects of oral administration of potassium and magnesium aspartate (K + Mg Asp) on physiologic responses to 90 min of treadmill walking at approximately 62% VO2 max were evaluated in seven healthy males (VO2 max = 59.5 ml X kg-1 X min-1). A total of 7.2 g of K + Mg Asp were administered to each subject during a 24 h period prior to work and compared to control and placebo trials. For control, placebo, and K + Mg Asp trials, no significant differences were observed in resting or exercise values for ventilation (VE), oxygen uptake (VO2), carbon dioxide production (VCO2), respiratory exchange ratio (RO), heart rate (HR), or blood pressure (BP). In addition, there were no differences between the three trials for exercise-induced decreases in body weight and increases in rectal temperature, or for pre- and post-exercise alterations in serum lactic acid, creatine kinase, lactic dehydrogenase, and percentage change in plasma volume. The findings from this study indicate that oral ingestion of K+ Mg Asp prior to exercise had no effect on cardiorespiratory, hematologic, and metabolic responses to 90 min of work conducted at approximately 62% VO2 max. /Potassium magnesium aspartate/ This study examined the effects of aspartate supplementation (ASP) on plasma ammonia concentrations (NH4+) during and after a resistance training workout (RTW). Twelve male weight trainers were randomly administered ASP or vitamin C in a crossover, double blind protocol, each trial separated by 1 wk. ASP and vitamin C were given over a 2 hr period beginning 5 hr prior to the RTW. The RTW consisted of bench, incline, shoulder, and triceps presses, and biceps curls at 70% of one repetition maximum (1-RM). After the RTW a bench press test (BPT) to failure at 65% of 1-RM was used to assess performance. (NH4+) was determined preexercise, 20 and 40 min midworkout, immediately postexercise, and 15 min postexercise. Treatment-by-time ANOVAs, paired t tests, and contrast comparisons were used to identify mean differences. No significant differences were observed between treatments for (NH4+) or BPT. (NH4+) increased significantly from Pre to immediately postexercise for both the ASP and vitamin C trials. Acute ASP supplementation does not reduce (NH4+) during and after a high intensity RTW in weight trained subjects. /Potassium magnesium aspartate/ Pharmacodynamics L-aspartate is considered a non-essential amino acid, meaning that, under normal physiological conditions, sufficient amounts of the amino acid are synthesized in the body to meet the body's requirements. L-aspartate is formed by the transamination of the Krebs cycle intermediate oxaloacetate. The amino acid serves as a precursor for synthesis of proteins, oligopeptides, purines, pyrimidines, nucleic acids and L-arginine. L-aspartate is a glycogenic amino acid, and it can also promote energy production via its metabolism in the Krebs cycle. These latter activities were the rationale for the claim that supplemental aspartate has an anti-fatigue effect on skeletal muscle, a claim that was never confirmed. |
| 分子式 |
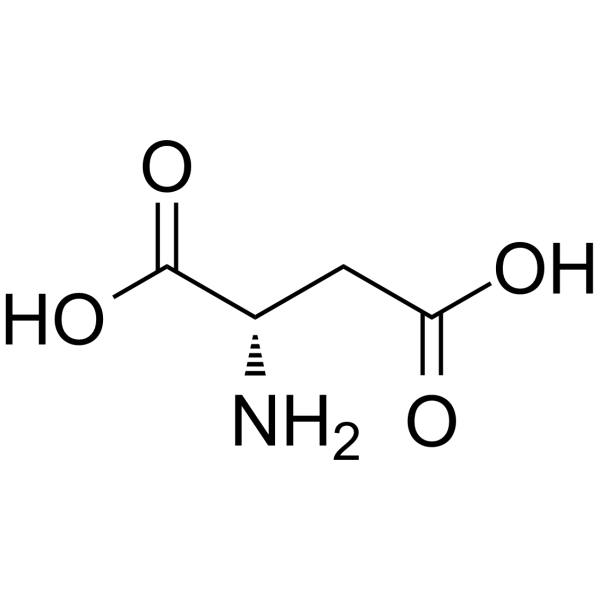
C4H7NO4
|
|---|---|
| 分子量 |
133.1027
|
| 精确质量 |
133.037
|
| CAS号 |
56-84-8
|
| 相关CAS号 |
25608-40-6;1115-63-5 (mono-potassium salt);14007-45-5 (potassium salt);17090-93-6 (hydrochloride salt);2001-89-0 (di-potassium salt);2068-80-6 (magnesium (2:1) salt);21059-46-1 (calcium salt);3792-50-5 (mono-hydrochloride salt);39162-75-9 (calcium (2:1) salt);5598-53-8 (di-hydrochloride salt)
|
| PubChem CID |
5960
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
264.1±30.0 °C at 760 mmHg
|
| 熔点 |
>300 °C (dec.)(lit.)
|
| 闪点 |
113.5±24.6 °C
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
| 折射率 |
1.531
|
| LogP |
-0.67
|
| tPSA |
100.62
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
9
|
| 分子复杂度/Complexity |
133
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C([C@@H](C(=O)O)N)C(=O)O
|
| InChi Key |
CKLJMWTZIZZHCS-REOHCLBHSA-N
|
| InChi Code |
InChI=1S/C4H7NO4/c5-2(4(8)9)1-3(6)7/h2H,1,5H2,(H,6,7)(H,8,9)/t2-/m0/s1
|
| 化学名 |
(2S)-2-aminobutanedioic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
1M NaOH : 100 mg/mL (~751.31 mM)
H2O : ~2 mg/mL (~15.03 mM) DMSO :< 1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 1 mg/mL (7.51 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。 (<60°C).
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.5131 mL | 37.5657 mL | 75.1315 mL | |
| 5 mM | 1.5026 mL | 7.5131 mL | 15.0263 mL | |
| 10 mM | 0.7513 mL | 3.7566 mL | 7.5131 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。