| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
阿托品(托品;1 μM;肺静脉和动脉)可抑制乙酰胆碱引起的人体肺静脉扩张[4]。
|
|---|---|
| 体内研究 (In Vivo) |
阿托品(托品;10 mg/kg;腹腔注射;40 分钟以上一次;Peromyscus sp.)通常发生在麻木期,可抑制心律失常[2]。
|
| 动物实验 |
Animal/Disease Models: White-footed mice (Peromyscus sp.) [2]
Doses: 10 mg/kg Route of Administration: intraperitoneal (ip) injection; once, lasting 40 minutes. Experimental Results: increased heart rate and diminished arrhythmia. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Hyoscyamine is completely absorbed by sublingual and oral routes, though exact data regarding the Cmax, Tmax, and AUC are not readily available. The majority of hyoscyamine is eliminated in the urine as the unmetabolized parent compound. Metabolism / Metabolites Hyoscyamine is largely unmetabolized, however a small amount is hydrolyzed into tropine and tropic acid. Biological Half-Life The half life of hyoscyamine is 3.5 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Despite widespread use over many decades, hyoscyamine has not been linked to episodes of liver enzyme elevations or clinically apparent liver injury. A major reason for its safety may relate to the low daily dose and limited duration of use. References on the safety and potential hepatotoxicity of anticholinergics are given together after the Overview section on Anticholinergic Agents. Drug Class: Gastrointestinal Agents; Anticholinergic Agents |
| 参考文献 | |
| 其他信息 |
Pharmacodynamics
Hyoscyamine is not FDA approved, and so it has not official indications. However, it is used as an antimuscarinic agent in a number of treatments and therapies. Hyoscyamine has a short duration of action as it may need to be given multiple times per day. Patients should be counselled regarding the risks and signs of anticholinergic toxicity. |
| 分子式 |
C17H23NO3
|
|---|---|
| 分子量 |
289.3694
|
| 精确质量 |
289.167
|
| CAS号 |
51-55-8
|
| 相关CAS号 |
Atropine sulfate monohydrate;5908-99-6;Atropine sulfate;55-48-1;(Rac)-Atropine-d3;1276197-36-4;Atropine hydrobromide;6415-90-3
|
| PubChem CID |
174174
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
429.8±45.0 °C at 760 mmHg
|
| 熔点 |
115-118 °C
|
| 闪点 |
213.7±28.7 °C
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
| 折射率 |
1.581
|
| LogP |
1.53
|
| tPSA |
49.77
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
21
|
| 分子复杂度/Complexity |
353
|
| 定义原子立体中心数目 |
2
|
| SMILES |
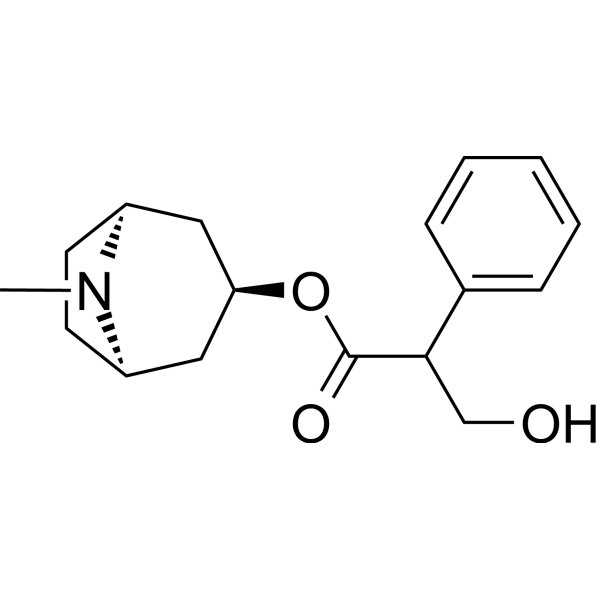
CN1[C@@H]2CC[C@H]1CC(C2)OC(=O)C(CO)C3=CC=CC=C3
|
| InChi Key |
RKUNBYITZUJHSG-PJPHBNEVSA-N
|
| InChi Code |
InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15?,16?
|
| 化学名 |
[(1R,5S)-8-methyl-8-azabicyclo[3.2.1]octan-3-yl] 3-hydroxy-2-phenylpropanoate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 96.6 mg/mL (~333.83 mM)
H2O : ~2.9 mg/mL (~10.02 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (7.19 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (7.19 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (7.19 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4558 mL | 17.2789 mL | 34.5578 mL | |
| 5 mM | 0.6912 mL | 3.4558 mL | 6.9116 mL | |
| 10 mM | 0.3456 mL | 1.7279 mL | 3.4558 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Topical Application of Low-concentration (0.01%) Atropine on the Human Eye With Fast and Slow Myopia Progression Rate
CTID: NCT03374306
Phase: N/A Status: Completed
Date: 2024-10-16
|