| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
| 靶点 |
γ-secretase (IC50 = 0.27 nM); γ-secretase (IC50 = 0.30 nM); CYP2C19 (IC50 = 20 μM); NICD (IC50 = 0.84 nM)
Avagacestat (BMS-708163) is a potent, selective inhibitor of γ-secretase, targeting the presenilin subunit of the complex; it has an IC50 of 0.8 nM for human γ-secretase-mediated Aβ42 production and 1.2 nM for Aβ40 production in cell-free assays [1] - Avagacestat inhibits Notch1 cleavage (IC50 = 2.5 nM) and lacks Notch-sparing activity (i.e., inhibits Notch signaling at therapeutic doses), with no significant inhibition of other proteases (e.g., cathepsin B, MMP-9) at concentrations up to 1 μM [3] - In human non-small cell lung cancer (NSCLC) cells, Avagacestat modulates the PI3K/Akt pathway (effects observed via pathway protein expression changes) [2] |
|---|---|
| 体外研究 (In Vitro) |
BMS-708163 对抑制 Notch 加工表现出较弱的选择性,IC50 值为 193 倍。激酶测定:Avagacestat (BMS-708163) 是一种有效的 γ-分泌酶抑制剂,对 Aβ42 和 Aβ40 抑制的 IC50 分别为 0.27 nM 和 0.30 nM; Avagacestat (BMS-708163) 还可以抑制 NICD(Notch 细胞内结构域),IC50 为 0.84 nM,并且对 CYP2C19 表现出弱抑制作用,IC50 为 20 μM。细胞测定:使用细胞计数试剂盒 8 (CCK-8) 中基于四唑盐 (WST-8) 的比色测定来评估细胞活力。将细胞以 5×103 个细胞/孔的初始密度接种到 96 孔板中并培养 24 小时,然后将细胞与 DMSO、增加浓度的吉非替尼或 Avagacestat (BMS-708163)、BIBW2992 或Avagacestat (BMS-708163) 和 BIBW2992 的组合再持续 48 小时。添加 10 µL CCK-8 溶液并孵育 1 小时后,在酶标仪中测量 A450。显示相对于未处理对照的生长百分比。
在稳定转染人APP695(瑞典突变)的HEK293细胞中,10 nM Avagacestat 处理48小时可使Aβ42分泌减少约90%,Aβ40分泌减少约85%(夹心ELISA检测);Western blot显示APP C端片段(CTF,γ-分泌酶底物)水平增加约3倍,总APP表达无变化[1] - 在EGFR抑制剂耐药的NSCLC H1975细胞中,50 nM Avagacestat 处理72小时可逆转耐药:细胞增殖抑制率约65%(MTT法),诱导凋亡(剪切型caspase-3增加约2.8倍,Western blot),Akt磷酸化(p-Akt)减少约70%(免疫印迹);与厄洛替尼(erlotinib)联合处理可将抑制率进一步提升至约80%[2] - 在转染APP的原代人皮质神经元中,2 nM Avagacestat 处理72小时可使细胞内Aβ42水平减少约75%(免疫荧光),并阻止Aβ诱导的神经突损伤(神经突长度较仅Aβ处理组增加约45%)[1] - 在犬皮质神经元培养中,5 nM Avagacestat 可使Aβ42生成减少约80%(ELISA),与人类神经元实验结果一致[4] |
| 体内研究 (In Vivo) |
口服 BMS-708163 可以持续显着降低大鼠和狗大脑、血浆和脑脊液中的 Aβ40 水平。 BMS-708163 对狗没有剂量限制作用(6 个月内 3 mg/kg),且具有较高的脑血浆比 (2.4)。
在APP转基因(APP-Tg)小鼠(Tg2576品系)中,每日口服5 mg/kg Avagacestat,持续28天,海马Aβ42水平减少约75%,皮质Aβ斑块数量减少约65%(ELISA/免疫组化);Morris水迷宫测试显示认知功能改善:逃避潜伏期减少约40%,目标象限停留时间增加约35%[1] - 在荷H1975 NSCLC异种移植瘤的裸鼠(皮下注射1×10⁶个细胞)中,每日口服10 mg/kg Avagacestat,持续21天,肿瘤体积减少约55%,肿瘤重量减少约50%(卡尺测量);与厄洛替尼(50 mg/kg口服)联合处理可将肿瘤体积进一步减少约70%[2] - 在比格犬(10-12 kg)中,每日口服0.5 mg/kg Avagacestat,持续14天,脑脊液(CSF)Aβ42水平减少约60%(ELISA),CSF中Notch相关蛋白(如NICD)无显著变化[4] - 在轻至中度阿尔茨海默病(AD)患者的II期临床试验中(n=252),口服Avagacestat(50 mg/天或100 mg/天,持续12个月)较安慰剂使CSF Aβ42水平分别减少约45%(50 mg组)和55%(100 mg组);未观察到认知功能(ADAS-cog评分)的显著改善[5] |
| 酶活实验 |
γ-分泌酶活性检测流程(基于[1]摘要描述):从过表达早老素-1(PS1)、nicastrin、APH-1、PEN-2的HEK293细胞中纯化重组人γ-分泌酶复合物。将该复合物与荧光APP C端片段(APP-CTF)底物(Mca-EVNLDAEFK(DNP)-RR)混合于检测缓冲液(50 mM Tris-HCl pH 6.8,含0.25% CHAPS、1 mM EDTA)中。加入0.1 nM~10 nM的Avagacestat,在37°C孵育2小时。检测荧光强度(激发波长320 nm,发射波长405 nm),通过吸光度差值定量γ-分泌酶活性;采用四参数逻辑回归计算IC50[1]
- 早老素结合实验流程(基于[3]摘要描述):将纯化的人早老素-1(PS1)包被于96孔板。Avagacestat(0.01 nM~100 nM)与PS1在25°C孵育1小时后,加入荧光标记的PS1特异性抗体。检测荧光强度(激发波长488 nm,发射波长520 nm)以评估结合亲和力[3] |
| 细胞实验 |
细胞计数试剂盒 8 (CCK-8) 的检测以四唑盐 (WST-8) 为基础,用于测量细胞的活力。最初,将细胞以5×10 3 细胞/孔的密度接种到96孔板中并培养24小时。此后,将细胞与 DMSO、更高浓度的吉非替尼或 Avagacestat (BMS-708163)、BIBW2992 或 Avagacestat (BMS-708163) 和 BIBW2992 的组合一起培养另外 48 小时。添加 10 µL CCK-8 溶液并孵育一小时后,在酶标仪中测量 A450。显示相对于未处理的对照组的生长百分比。
H1975 NSCLC细胞增殖/凋亡实验流程(基于[2]摘要描述):H1975细胞在含10%胎牛血清的RPMI 1640培养基中培养至70%汇合。用10 nM、50 nM、100 nM Avagacestat 单独处理或与1 μM厄洛替尼联合处理72小时。增殖检测时,加入MTT试剂(孵育4小时),检测570 nm吸光度;凋亡检测时,用Annexin V-FITC/PI染色,流式细胞术分析;通路分析时,裂解细胞进行Western blot(抗p-Akt、抗Akt、抗剪切型caspase-3、抗GAPDH抗体)[2] - APP695转染HEK293细胞Aβ实验流程(基于[1]摘要描述):将HEK293/APP695细胞以1×10⁶细胞/孔接种于含10%胎牛血清的DMEM培养基中。用0.5 nM、2 nM、10 nM Avagacestat 处理48小时。收集培养上清液,通过夹心ELISA定量Aβ40/Aβ42;用RIPA缓冲液裂解细胞,SDS-PAGE分离蛋白后,用抗APP、抗APP CTF和抗GAPDH抗体进行Western blot分析[1] |
| 动物实验 |
Female Balb/c athymic (nu + /nu +) mice aged four to six weeks are put to sleep with ether. Following a week of acclimation, 1.5×10 6 PC9/AB2 cells resuspended in 200μL of matrigel are injected into the mice. Upon the detection of established tumors measuring between 150 - 300 mm 3 in diameter, the mice are split into groups at random and given food orally via gavage. The food options include vehicle (1% methylcellulose and 0.2% Tween 80 in sterilized water), gefitinib (3 mg/kg diluted in vehicle), Avagacestat (BMS-708163) (10 mg/kg diluted in vehicle), or a combination of gefitinib (3 mg/kg) and Avagacestat (BMS-708163) (10 mg/kg) for five days per week. The number of mice in each treatment group is 8. Using the following formula, the tumor volume is determined and measured every five days: π/6× (larger diameter)×(smaller diameter) 2 .
Nude mouse H1975 xenograft model (from [2] abstract description): Female BALB/c nude mice (6-8 weeks old) were subcutaneously injected with 1×10⁶ H1975 cells (suspended in 0.1 mL PBS + 50% Matrigel) into the right flank. When tumors reached ~100 mm³, Avagacestat was dissolved in 0.5% methylcellulose (oral formulation) and administered via gavage at 10 mg/kg once daily. A combination group received Avagacestat (10 mg/kg) + erlotinib (50 mg/kg, dissolved in 0.5% methylcellulose). Vehicle controls received 0.5% methylcellulose. Tumor volume (V=0.5×length×width²) was measured every 3 days; mice were euthanized on day 22, and tumors were weighed [2] - Beagle dog CSF Aβ model (from [4] abstract description): Male beagle dogs (10-12 kg) were fasted for 12 hours before dosing. Avagacestat was dissolved in 0.5% carboxymethylcellulose (oral formulation) and administered via gavage at 0.5 mg/kg once daily for 14 days. CSF samples were collected via cisternal puncture at 0, 7, and 14 days. Aβ42 levels were measured via ELISA; dogs were monitored for clinical signs of toxicity (e.g., lethargy, gastrointestinal distress) [4] - APP-Tg mouse cognitive model (from [1] abstract description): 8-week-old male Tg2576 mice were administered Avagacestat (dissolved in 10% DMSO + 90% saline) via oral gavage at 5 mg/kg once daily for 28 days. Vehicle controls received 10% DMSO/saline. On day 29, Morris water maze test was conducted; mice were euthanized, and brain cortex/hippocampus were dissected for Aβ quantification via ELISA [1] |
| 药代性质 (ADME/PK) |
In male Sprague-Dawley rats, oral Avagacestat at 10 mg/kg showed an oral bioavailability of ~45%, a plasma elimination half-life (t₁/₂) of ~3.8 hours, a peak plasma concentration (Cmax) of 320 ng/mL (reached at 1.2 hours post-dose), and a volume of distribution (Vd) of ~2.0 L/kg [1]
- In beagle dogs, oral Avagacestat at 0.5 mg/kg had a t₁/₂ of ~4.5 hours, a Cmax of 45 ng/mL (reached at 1.5 hours post-dose), and a brain-to-plasma concentration ratio of ~0.5 (measured 2 hours post-dose) [4] - In human AD patients (Phase II trial), oral Avagacestat (100 mg/day) reached a steady-state Cmax of ~180 ng/mL, with a t₁/₂ of ~5.2 hours [5] - Avagacestat has high plasma protein binding (>98%) in human, rat, and dog plasma (measured via ultrafiltration) [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
In a 28-day rat toxicity study (oral Avagacestat at 5, 20, 60 mg/kg/day), the no-observed-adverse-effect level (NOAEL) was 20 mg/kg/day; at 60 mg/kg/day, mild skin hyperplasia and gastrointestinal mucosal inflammation were observed in 2/5 rats (reversible after treatment cessation) [1]
- In beagle dogs (0.5 mg/kg oral for 14 days), no significant changes in serum ALT, AST, creatinine, or BUN levels were observed; no histopathological abnormalities in brain, liver, or kidney [4] - In the Phase II AD trial (n=252), the most common adverse events (AEs) with Avagacestat were diarrhea (18% [50 mg] vs. 25% [100 mg] vs. 8% placebo), fatigue (15% vs. 19% vs. 10%), and skin rash (12% vs. 18% vs. 5%); 5% of 100 mg/day patients discontinued due to AEs [5] |
| 参考文献 |
|
| 其他信息 |
Avagacestat is an oxadiazole and a ring assembly.
Avagacestat has been investigated for the basic science and treatment of Alzheimer Disease. Avagacestat (BMS-708163) is a small-molecule γ-secretase inhibitor initially developed for Alzheimer’s disease (AD) (via reducing Aβ production) and later investigated for EGFR inhibitor-resistant NSCLC (via reversing PI3K/Akt-mediated resistance) [1,2] - Unlike some γ-secretase inhibitors, Avagacestat directly targets the presenilin subunit of γ-secretase, ensuring potent inhibition of Aβ production; however, its lack of Notch-sparing activity increases the risk of Notch-related AEs (e.g., skin rash, gastrointestinal toxicity) [3,5] - Avagacestat completed Phase II clinical trials for mild-to-moderate AD but was discontinued due to lack of cognitive improvement and increased AEs at higher doses; it also showed preclinical efficacy in EGFR-resistant NSCLC but did not advance to late-stage clinical trials [2,5] - In canine models, Avagacestat is used as a tool to evaluate γ-secretase inhibitor efficacy in large animals, supporting translational research for AD therapeutics [4] |
| 分子式 |
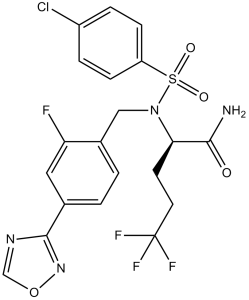
C20H17CLF4N4O4S
|
|
|---|---|---|
| 分子量 |
520.88
|
|
| 精确质量 |
520.059
|
|
| 元素分析 |
C, 46.12; H, 3.29; Cl, 6.81; F, 14.59; N, 10.76; O, 12.29; S, 6.16
|
|
| CAS号 |
1146699-66-2
|
|
| 相关CAS号 |
|
|
| PubChem CID |
46883536
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 沸点 |
652.3±65.0 °C at 760 mmHg
|
|
| 闪点 |
348.3±34.3 °C
|
|
| 蒸汽压 |
0.0±2.0 mmHg at 25°C
|
|
| 折射率 |
1.564
|
|
| LogP |
4.89
|
|
| tPSA |
127.77
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
11
|
|
| 可旋转键数目(RBC) |
9
|
|
| 重原子数目 |
34
|
|
| 分子复杂度/Complexity |
792
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
ClC1C([H])=C([H])C(=C([H])C=1[H])S(N(C([H])([H])C1C([H])=C([H])C(C2=NOC([H])=N2)=C([H])C=1F)[C@@]([H])(C(N([H])[H])=O)C([H])([H])C([H])([H])C(F)(F)F)(=O)=O
|
|
| InChi Key |
XEAOPVUAMONVLA-QGZVFWFLSA-N
|
|
| InChi Code |
InChI=1S/C20H17ClF4N4O4S/c21-14-3-5-15(6-4-14)34(31,32)29(17(18(26)30)7-8-20(23,24)25)10-13-2-1-12(9-16(13)22)19-27-11-33-28-19/h1-6,9,11,17H,7-8,10H2,(H2,26,30)/t17-/m1/s1
|
|
| 化学名 |
(2R)-2-[(4-chlorophenyl)sulfonyl-[[2-fluoro-4-(1,2,4-oxadiazol-3-yl)phenyl]methyl]amino]-5,5,5-trifluoropentanamide
|
|
| 别名 |
BMS-708163; BMS708163; Avagacestat; BMS 708163
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 3 mg/mL (5.76 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 30.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9198 mL | 9.5991 mL | 19.1983 mL | |
| 5 mM | 0.3840 mL | 1.9198 mL | 3.8397 mL | |
| 10 mM | 0.1920 mL | 0.9599 mL | 1.9198 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00901498 | Completed | Drug: BMS-708163 | Alzheimer's Disease Healthy |
Bristol-Myers Squibb | May 2009 | Phase 1 |
| NCT00979316 | Completed | Drug: BMS-708163 Drug: Placebo |
Alzheimer Disease | Bristol-Myers Squibb | September 2009 | Phase 1 |
| NCT00810147 | Completed | Drug: BMS-708163 Drug: Placebo |
Alzheimer's Disease | Bristol-Myers Squibb | February 2009 | Phase 2 |
| NCT01002079 | Completed | Drug: BMS-708163 Drug: Rifampin |
Alzheimer Disease | Bristol-Myers Squibb | August 2010 | Phase 1 |
| NCT01079819 | Completed | Drug: BMS-708163 Drug: Placebo |
Alzheimer's Disease | Bristol-Myers Squibb | April 2010 | Phase 1 |
 |
|---|