| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Proteasome (IC50 = 5 nM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:Carfilzomib 抑制多种细胞系和患者来源的肿瘤细胞(包括多发性骨髓瘤)的增殖,并诱导内在和外在的细胞凋亡信号通路以及 c-Jun-N 末端激酶 (JNK) 的激活。与硼替佐米相比,卡非佐米具有增强的抗 MM 活性,克服了对硼替佐米和其他药物的耐药性,并与地塞米松 (Dex) 具有协同作用。卡非佐米对 β5 亚基中的 ChT-L 活性具有优先的体外抑制效力,在 10 nM 剂量下抑制率超过 80%。短时间暴露于低剂量卡非佐米会导致β5组成型20S蛋白酶体和β5i免疫蛋白酶体亚基的优先结合特异性。用卡非佐米脉冲的 ANBL-6 细胞中 caspase 活性的测量显示,8 小时后 caspase-8、caspase-9 和 caspase-3 活性显着增加,分别比对照增加 3.2、3.9 和 6.9 倍8小时后的细胞。在卡非佐米脉冲处理的细胞中,线粒体膜完整性降低至 41% (Q1 + Q2),而在媒介物处理的对照细胞中为 75%。在另一项研究中,卡非佐米还显示出针对血液系统和实体恶性肿瘤的临床前有效性。卡非佐米直接抑制破骨细胞形成和骨吸收。激酶测定:将 ANBL-6 细胞(2 × 106/孔)接种于 96 孔板中,并用 0.001 至 10 μM 剂量的卡非佐米处理 1 小时。然后裂解细胞(20 mM Tris-HCl、0.5 mM EDTA),并将澄清的裂解物转移至聚合酶链式反应 (PCR) 板。使用未处理的 ANBL-6 细胞裂解物生成标准曲线,起始浓度为 6 μg 蛋白质/μL。活性位点探针[生物素-(CH2)4-Leu-Leu-Leu-环氧酮;添加20 μM]并在室温下孵育1小时。然后通过添加 1% 十二烷基硫酸钠 (SDS) 并加热至 100°C 使细胞裂解物变性,然后在 96 孔多屏 DV 板中与每孔 20 μL 链霉亲和素-琼脂糖高性能珠混合并孵育 1 小时。然后用酶联免疫吸附测定 (ELISA) 缓冲液(PBS、1% 牛血清白蛋白和 0.1% Tween-20)洗涤这些珠子,并在平板摇床上与蛋白酶体亚基抗体在 4°C 下孵育过夜。使用的抗体包括小鼠单克隆抗β1、抗β2、抗β1i和抗β5i、山羊多克隆抗β2i和兔多克隆抗β5(针对KLH-CWIRVSSDNVADLHDKYS肽的亲和纯化抗血清)。洗涤珠子并与辣根过氧化物酶缀合的二抗山羊抗兔、山羊抗小鼠或兔抗山羊抗体一起孵育 2 小时。清洗后,使用 Supersignal ELISA 皮化学发光底物对珠子进行显色。进行发光检测。通过与标准曲线比较,将原始发光转换为μg/mL,并表示为相对于载体对照的抑制百分比。使用以下非 S 型剂量反应方程生成曲线拟合:Y = Bottom + (Top-Bottom)/(1 + 10̂((LogEC50 − X) × HillSlope)),其中 X 是浓度的对数,Y 是 %抑制作用,EC50是显示50%效果的剂量。细胞测定:WST-1 用于测定蛋白酶体抑制剂卡非佐米对细胞增殖的影响。相对于仅接受媒介物的平行对照细胞计算增殖抑制。使用 XLfit 4 软件,使用线性样条函数插值中值抑制浓度 (IC50)。耐药程度(DOR)的计算公式为:DOR = IC50(耐药细胞)/IC50(敏感细胞)。将用 100 nM 卡非佐米脉冲的 ANBL-6 细胞洗涤并悬浮在含有 5 μg/mL JC-1 的 PBS 中,JC-1 在线粒体中表现出电位依赖性积累。在 FacScan 上对从 525 nm 到 590 nm 的线粒体膜电位依赖性色移进行分析,并使用 CellQuest 软件对数据进行分析。
|
| 体内研究 (In Vivo) |
卡非佐米在体内异种移植模型中适度降低肿瘤生长。在连续或短暂的模拟治疗后,卡非佐米可有效降低多发性骨髓瘤细胞的活力。卡非佐米可增加非肿瘤小鼠的骨小梁体积,减少骨吸收并增强骨形成。
|
| 酶活实验 |
ANBL-6 细胞(以 2 × 106/孔铺板)接受卡非佐米(Carfilzomib)处理 1 小时,剂量范围为 0.001 至 10 μM。下一步涉及裂解细胞(20 mM Tris-HCl、0.5 mM EDTA),然后将澄清的裂解物置于 PCR 板上。使用未经处理的 ANBL-6 细胞裂解物创建标准曲线,浓度为 6 μg 蛋白质/μL。添加活性位点探针(生物素-(CH2)4-Leu-Leu-Leu-环氧酮;20 μM)后,将混合物在室温下孵育一小时。将细胞裂解物加热至 100°C 并添加 1% 十二烷基硫酸钠 (SDS) 后,将混合物与 96 孔多屏 DV 板中每孔 20 μL 的链霉亲和素-琼脂糖高性能珠混合,并孵育混合物一个小时。在含有 PBS、1% 牛血清白蛋白和 0.1% Tween-20 的溶液中洗涤珠子后,将珠子与抗蛋白酶体亚基的抗体在平板摇床上于 4°C 下孵育整晚。使用的抗体包括山羊多克隆抗-β2i、兔多克隆抗-β5(针对KLH-CWIRVSSDNVADLHDKYS肽的亲和纯化抗血清)和小鼠单克隆抗-β1、抗-β2、抗-β1i和抗-β5i。将山羊抗兔、山羊抗小鼠或与辣根过氧化物酶缀合的兔抗山羊二抗应用于珠子,然后孵育 2 小时。 Supersignal ELISA 皮化学发光底物用于在清洗后对珠子进行显色。一种进行发光检测。原始发光表示为与载体对照相比的抑制百分比,并通过与标准曲线比较转换为 μg/mL。以下非 S 形剂量反应方程用于创建曲线拟合:Y = Bottom + (Top-Bottom)/(1 + 10̂((LogEC50 − X) × HillSlope)),其中 EC50 是表现出 50% 效应的剂量,X为浓度的对数,Y为抑制百分比。
|
| 细胞实验 |
WST-1 用于评估蛋白酶体抑制剂卡非佐米如何影响细胞生长。增殖抑制的计算基于单独给予媒介物的平行对照细胞。 XLfit 4 软件用于使用线性样条函数插值中值抑制浓度 (IC50)。使用以下公式确定耐药程度(DOR):DOR = IC50(耐药细胞)/IC50(敏感细胞)。用 100 nM 卡非佐米脉冲后,清洁 ANBL-6 细胞并将其悬浮在含有 5 μg/mL JC-1 的 PBS 中,JC-1 是一种以电位依赖性方式在线粒体中积累的酶。使用 FacScan,检查从 525 到 590 nm 的线粒体膜电位依赖性色移。 CellQuest 软件用于分析数据。
|
| 动物实验 |
Beige-nude-XID mice are used in animal research. After pelleting 10×106 Granta514 cells and twice washing them in 1X PBS, the cells are subcutaneously injected into the right flank. Following the appearance of tumors, carfilzomib-vorinostat is administered to five to six mice, and the growth or regression of the tumors is tracked throughout treatment. In DMSO and 10% sulfobutylether betacyclodextrin at a pH of 10 mM citrate buffer, stock vorinostat and carfilzomib are dissolved, respectively. Before injection, they are diluted and kept in small aliquots at -80°C for storage.
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Cmax, single IV dose of 27 mg/m^2 = 4232 ng/mL; AUC, single IV dose of 27 mg/m^2 = 379 ng•hr/mL; Carfilzomib does not accumulation in the systemic. At doses between 20 and 36 mg/m2, there was a dose-dependent increase in exposure. Vd, steady state, 20 mg/m^2 = 28 L Systemic clearance = 151 - 263 L/hour. As this value exceeds hepatic blood flow, it suggests that carfilozmib is cleared extrahepatically. Metabolism / Metabolites Carfilzomib was rapidly and extensively metabolized by the liver. The predominant metabolites were the peptide fragments and the diol of carfilzomib which suggests that the main metabolic pathways are peptidase cleavage and epoxide hydrolysis. The cytochrome P450 enzyme system is minimally involved in the metabolism of carfilzomib. All metabolites are inactive. Biological Half-Life Following intravenous administration of doses ≥ 15 mg/m^2, carfilzomib was rapidly cleared from the systemic circulation with a half-life of ≤ 1 hour on Day 1 of Cycle 1. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In large clinical trials of carfilzomib, elevations in serum aminotransferase levels were common, occurring in 8% to 13% of patients. However, values greater than 5 times the upper limit of normal (ULN) were uncommon, occurring in 1% to 2% of recipients. In several studies there were reports of clinically apparent liver injury including acute liver failure in patients receiving carfilzomib; however, in most instances multiple concomitant medications were being taken (such as lenalidomide) and the specific role of carfilzomib in causing the liver injury was not always clear. The onset of injury was typically during the first cycle of therapy. The clinical features and pattern of injury in clinically apparent cases of liver injury due to carfilzomib have not been described in the published literature. Hepatotoxicity is listed as a warning in the product label for carfilzomib and monitoring of serum enzymes during treatment is recommended. Likelihood score: D (Possible cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of carfilzomib during breastfeeding. Because carfilzomib is 97% bound to plasma proteins, the amount in milk is likely to be low. The manufacturer recommends that breastfeeding be discontinued during carfilzomib therapy and for 2 weeks after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Over the concentration range of 0.4 - 4 micromolar, carfilzomib was 97% protein bound. |
| 参考文献 | |
| 其他信息 |
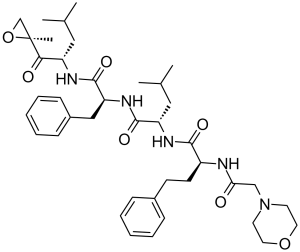
Carfilzomib is a synthetic tetrapeptide consisting of morpholin-4-acetyl, L-2-amino-4-phenylbutanoyl, L-leucyl and L-phenylalanyl residues joined in sequence with the C-terminus connected to the amino group of (2S)-2-amino-4-methyl-1-[(2R)-2-methyloxiran-2-yl]-1-oxopentan-1-one via an amide linkage. Used for the treatment of patients with multiple myeloma It has a role as an antineoplastic agent and a proteasome inhibitor. It is a tetrapeptide, a member of morpholines and an epoxide.
Carfilzomib is an injectable antineoplastic agent (IV only). Chemically, it is a modified tetrapeptidyl epoxide and an analog of epoxomicin. It is also a selective proteasome inhibitor. FDA approved carfilzomib in July 2012 for the treatment of adults with relapsed or refractory multiple myeloma as monotherapy or combination therapy. Carfilzomib is a Proteasome Inhibitor. The mechanism of action of carfilzomib is as a Proteasome Inhibitor. Carfilzomib is an irreversible proteasome inhibitor and antineoplastic agent that is used in treatment of refractory multiple myeloma. Carfilzomib is associated with a low rate of serum enzyme elevations during treatment and has been implicated to rare instances of clinically apparent, acute liver injury some of which have been fatal. Carfilzomib is an epoxomicin derivate with potential antineoplastic activity. Carfilzomib irreversibly binds to and inhibits the chymotrypsin-like activity of the 20S catalytic core subunit of the proteasome, a protease complex responsible for degrading a large variety of cellular proteins. Inhibition of proteasome-mediated proteolysis results in an accumulation of polyubiquinated proteins, which may lead to cell cycle arrest, induction of apoptosis, and inhibition of tumor growth. Drug Indication Carfilzomib is indicated for the treatment of adult patients with relapsed or refractory multiple myeloma who have received one to three lines of therapy in combination with lenalidomide and dexamethasone; or dexamethasone; or daratumumab and dexamethasone; or daratumumab and hyaluronidase-fihj and dexamethasone; or isatuximab and dexamethasone. It is also indicated as a single agent for the treatment of patients with relapsed or refractory multiple myeloma who have received one or more lines of therapy. FDA Label Kyprolis in combination with daratumumab and dexamethasone, with lenalidomide and dexamethasone, or with dexamethasone alone is indicated for the treatment of adult patients with multiple myeloma who have received at least one prior therapy. Treatment of acute lymphoblastic leukaemia Treatment of Multiple Myeloma Mechanism of Action Carfilzomib is made up of four modified peptides and acts as a proteasome inhibitor. Carfilzomib irreversibly and selectively binds to N-terminal threonine-containing active sites of the 20S proteasome, the proteolytic core particle within the 26S proteasome. This 20S core has 3 catalytic active sites: the chymotrypsin, trypsin, and caspase-like sites. Inhibition of the chymotrypsin-like site by carfilzomib (β5 and β5i subunits) is the most effective target in decreasing cellular proliferation, ultimately resulting in cell cycle arrest and apoptosis of cancerous cells. At higher doses, carfilzomib will inhibit the trypsin-and capase-like sites. Pharmacodynamics Intravenous carfilzomib administration resulted in suppression of proteasome chymotrypsin-like activity when measured in blood 1 hour after the first dose. On Day 1 of Cycle 1, proteasome inhibition in peripheral blood mononuclear cells (PBMCs) ranged from 79% to 89% at 15 mg/m2, and from 82% to 83% at 20 mg/m2. In addition, carfilzomib administration resulted in inhibition of the LMP2 and MECL1 subunits of the immunoproteasome ranging from 26% to 32% and 41% to 49%, respectively, at 20 mg/m2. Proteasome inhibition was maintained for ≥ 48 hours following the first dose of carfilzomib for each week of dosing. Resistance against carfilzomib has been observed and although the mechanism has not been confirmed, it is thought that up-regulation of P-glycoprotein may be a contributing factor. Furthermore, studies suggest that carfilzomib is more potent than bortezomib. |
| 分子式 |
C40H57N5O7
|
|---|---|
| 分子量 |
719.91
|
| 精确质量 |
719.425
|
| 元素分析 |
C, 66.73; H, 7.98; N, 9.73; O, 15.56
|
| CAS号 |
868540-17-4
|
| 相关CAS号 |
Carfilzomib-d8;1537187-53-3
|
| PubChem CID |
11556711
|
| 外观&性状 |
White solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
975.6±65.0 °C at 760 mmHg
|
| 熔点 |
204-208°C
|
| 闪点 |
543.8±34.3 °C
|
| 蒸汽压 |
0.0±0.3 mmHg at 25°C
|
| 折射率 |
1.551
|
| LogP |
6.71
|
| tPSA |
172.43
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
20
|
| 重原子数目 |
52
|
| 分子复杂度/Complexity |
1180
|
| 定义原子立体中心数目 |
5
|
| SMILES |
C([C@@]1(OC1)C)(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CN1CCOCC1)CCC1C=CC=CC=1)CC1C=CC=CC=1
|
| InChi Key |
BLMPQMFVWMYDKT-NZTKNTHTSA-N
|
| InChi Code |
InChI=1S/C40H57N5O7/c1-27(2)22-32(36(47)40(5)26-52-40)42-39(50)34(24-30-14-10-7-11-15-30)44-38(49)33(23-28(3)4)43-37(48)31(17-16-29-12-8-6-9-13-29)41-35(46)25-45-18-20-51-21-19-45/h6-15,27-28,31-34H,16-26H2,1-5H3,(H,41,46)(H,42,50)(H,43,48)(H,44,49)/t31-,32-,33-,34-,40+/m0/s1
|
| 化学名 |
(2S)-4-methyl-N-[(2S)-1-[[(2S)-4-methyl-1-[(2R)-2-methyloxiran-2-yl]-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]-2-[[(2S)-2-[(2-morpholin-4-ylacetyl)amino]-4-phenylbutanoyl]amino]pentanamide
|
| 别名 |
PR-171; PR 171; PR171; Carflizomib; brand name: Kyprolis
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 该产品在溶液状态不稳定,请现配现用。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (3.47 mM) = in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (3.47 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。= View More
配方 3 中的溶解度: 2.5 mg/mL (3.47 mM) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 配方 4 中的溶解度: 2% DMSO+castor oil: 10 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3891 mL | 6.9453 mL | 13.8906 mL | |
| 5 mM | 0.2778 mL | 1.3891 mL | 2.7781 mL | |
| 10 mM | 0.1389 mL | 0.6945 mL | 1.3891 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Subcutaneous Daratumumab, Once Weekly Carfilzomib, and Dexamethasone (DKd) in Patients With High-Risk Smoldering Multiple Myeloma
CTID: NCT04933539
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-11-25