| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
20S proteasome β5 (IC50 = 3.4 nM); 20S proteasome β1 (IC50 = 31 nM); 20S proteasome β2 (IC50 = 3500 nM)
|
|---|---|
| 体外研究 (In Vitro) |
Ixazomib citrate (MLN9708; 0.20-3.20 μM) 以时间和剂量依赖性方式有效抑制两种细胞系的生长。 ixazomib 在 MG-63 和 Saos-2 细胞中诱导细胞周期停滞。 Ixazomib 需要激活 caspase8 和 caspase9 才能主要通过 caspase 途径诱导细胞凋亡。 ixazomib 治疗可提高促凋亡蛋白水平并降低控制 MOMP 的抗凋亡蛋白水平。 ixazomib 治疗导致线粒体释放 Cytc、Smac 和 OMI,并降低 XIAP 蛋白水平。 Ixazomib 降低 MMP2/9 的表达和分泌水平,并抑制 MG-63 和 Saos-2 细胞的侵袭能力[1]。 Ixazomib citrate (MLN9708; 12 nM) 对 TL 和 CL 蛋白酶体的活性具有抑制作用。 Ixazomib 处理 H929 和 MM.1S MM 细胞会导致聚 (ADP) 核糖聚合酶 (PARP) 蛋白水解裂解显着增加,这是细胞凋亡过程中的标志性事件。上游 PARP 激活剂 caspase-3 被 isxazomib 裂解。 Ixazomib 增加 CHOP/GADD153 和 Bip 蛋白的水平以及 eIf2-α 激酶活性。 Ixazomib 靶向 NF-κB,在体外抑制毛细管形成,并阻断 BMSC 诱导的 MM 细胞增殖 [2]。
|
| 体内研究 (In Vivo) |
Ixazomib citrate (MLN9708;11 mg/kg) 通过显着阻止 MM 肿瘤的生长来提高人浆细胞瘤 MM.1S 异种移植小鼠模型的存活率。使用伊沙佐米治疗的小鼠血液化学特征中的血红蛋白、胆红素和肌酐水平均正常。 ixazomib 显着增加异种移植模型中克隆的 caspase-3 阳性细胞数[2]。
|
| 细胞实验 |
MTT 测定用于确定细胞活力。经胰蛋白酶消化的细胞以每孔 5000 个接种于 96 孔板中。将 Ixazomib 或 DMSO 添加到基础培养基中,并按规定的时间和剂量给予细胞。相对于单独给予媒介物的对照细胞来评估细胞的活力。
|
| 动物实验 |
Ixazomib is dissolved at a concentration of 2 mg/mL in 5% 2-hydroxypropyl-β-cyclodextrin. The test makes use of a human plasmacytoma xenograft tumor model. After receiving a subcutaneous inoculation of 5.0×106 MM.1S cells in 100 µL serum-free RPMI-1640 medium, 21 CB-17 SCID mice are randomly assigned to treatment groups once their tumors have grown to a size of 250–300 mm3. For three weeks, mice are given vehicle, bortezomib (1 mg/kg; i.v.) or ixazomib (11 mg/kg; i.v.) twice a week. When a tumor grows to be 2 cm3, the animal is put to death.
|
| 参考文献 |
|
| 其他信息 |
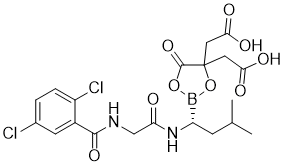
Ixazomib citrate is a glycine derivative that is the amide obtained by formal condensation of the carboxy group of N-(2,5-dichlorobenzoyl)glycine with the amino group of 2,2'-{2-[(1R)-1-amino-3-methylbutyl]-5-oxo-1,3,2-dioxaborolane-4,4-diyl}diacetic acid. A prodrug for ixazomib that is used in combination therapy for treatment of multiple myeloma. It has a role as a prodrug, a proteasome inhibitor, an orphan drug, an antineoplastic agent and an apoptosis inducer. It is a glycine derivative, a member of benzamides, a dichlorobenzene, an oxo dicarboxylic acid and a 1,3,2-dioxaborolane. It is functionally related to an ixazomib.
Ixazomib Citrate is the citrate salt form of ixazomib, an orally bioavailable second generation proteasome inhibitor (PI) with potential antineoplastic activity. Ixazomib inhibits the activity of the proteasome, blocking the targeted proteolysis normally performed by the proteasome, which results in an accumulation of unwanted or misfolded proteins; disruption of various cell signaling pathways may follow, resulting in the induction of apoptosis. Compared to first generation PIs, second generation PIs may have an improved pharmacokinetic profile with increased potency and less toxicity. Proteasomes are large protease complexes that degrade unneeded or damaged proteins that have been ubiquinated. See also: Ixazomib (has active moiety). Drug Indication Ninlaro in combination with lenalidomide and dexamethasone is indicated for the treatment of adult patients with multiple myeloma who have received at least one prior therapy. Treatment of systemic light chain amyloidosis |
| 分子式 |
C₂₀H₂₃BCL₂N₂O₉
|
|---|---|
| 分子量 |
517.12
|
| 精确质量 |
516.087
|
| 元素分析 |
C, 46.45; H, 4.48; B, 2.09; Cl, 13.71; N, 5.42; O, 27.85
|
| CAS号 |
1239908-20-3
|
| 相关CAS号 |
Ixazomib;1072833-77-2
|
| PubChem CID |
56844015
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 折射率 |
1.580
|
| LogP |
3.378
|
| tPSA |
175.31
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
11
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
797
|
| 定义原子立体中心数目 |
1
|
| SMILES |
ClC1C([H])=C([H])C(=C([H])C=1C(N([H])C([H])([H])C(N([H])[C@]([H])(B1OC(C(C([H])([H])C(=O)O[H])(C([H])([H])C(=O)O[H])O1)=O)C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H])=O)=O)Cl
|
| InChi Key |
MBOMYENWWXQSNW-AWEZNQCLSA-N
|
| InChi Code |
InChI=1S/C20H23BCl2N2O9/c1-10(2)5-14(21-33-19(32)20(34-21,7-16(27)28)8-17(29)30)25-15(26)9-24-18(31)12-6-11(22)3-4-13(12)23/h3-4,6,10,14H,5,7-9H2,1-2H3,(H,24,31)(H,25,26)(H,27,28)(H,29,30)/t14-/m0/s1
|
| 化学名 |
2-[4-(carboxymethyl)-2-[(1R)-1-[[2-[(2,5-dichlorobenzoyl)amino]acetyl]amino]-3-methylbutyl]-5-oxo-1,3,2-dioxaborolan-4-yl]acetic acid
|
| 别名 |
Ninlaro; MLN9708; MLN 9708; MLN-9708; ixazomib citrate; MMLN 2238-prodrug; MMLN-2238-prodrug; MMLN2238-prodrug; Ixazomib-prodrug
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 100~250 mg/mL (193.4~483.5 mM)
Ethanol: ~100 mg/mL (~193.4 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.02 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.02 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.02 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9338 mL | 9.6689 mL | 19.3379 mL | |
| 5 mM | 0.3868 mL | 1.9338 mL | 3.8676 mL | |
| 10 mM | 0.1934 mL | 0.9669 mL | 1.9338 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Venetoclax, Ixazomib Citrate, and Dexamethasone in Treating Patients with Relapsed Multiple Myeloma
CTID: NCT03399539
Phase: Phase 1 Status: Completed
Date: 2024-11-04