| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
Bcr-Abl (IC50 = 1.0 nM); Src (IC50 = 0.5 nM); lck (IC50 = 0.4 nM); yes (IC50 = 0.5 nM); c-kit (IC50 = 5.0 nM); PDGFRβ (IC50 = 28 nM); p38 (IC50 = 100 nM); Her1 (IC50 = 180 nM); Her2 (IC50 = 710 nM); FGFR-1 (IC50 = 880 nM); MEK (IC50 = 1700 nM)
Src Kinase (IC50 = 0.5 nM), Abl Kinase (wild-type, IC50 = 0.6 nM); no significant activity against EGFR, HER2 (IC50 > 1000 nM) [1a] - Imatinib-resistant Abl mutants: Abl Y253F (IC50 = 1.8 nM), Abl E255K (IC50 = 2.2 nM), Abl T315I (IC50 = 3.5 nM) [1b] - KIT Kinase (D816V mutant, IC50 = 3.0 nM); no activity against wild-type KIT (IC50 = 280 nM) [2] |
|---|---|
| 体外研究 (In Vitro) |
达沙替尼针对 Bcr-Abl、Src、Lck、Yes、c-Kit、PDGFRβ、p38、Her1、Her2、FGFR-1 和 MEK 表现出显着的作用,IC50 值小于 1.0、0.50、0.40、0.50、5.0分别为 28、100、180、720、880 和 1700 nM[1]。与 K562 慢性粒细胞白血病 (CML)、PC3 人前列腺肿瘤、MDA-MB-231 人乳腺肿瘤和 WiDr 人结肠癌细胞相比,达沙替尼的 IC50 分别小于 1.0 nM 和 9.4 nM线。 52 nM 和 12 nM[1]。
抑制Bcr-Abl阳性细胞增殖:慢性髓系白血病(CML)K562细胞(IC50 = 1.2 nM);10 nM Dasatinib(BMS354825; Sprycel)处理14天,K562细胞集落形成减少90%[1a] - 抑制伊马替尼耐药CML细胞:表达Abl Y253F的细胞(IC50 = 2.5 nM)、表达Abl E255K的细胞(IC50 = 3.1 nM);50 nM Dasatinib处理耐药细胞2小时,p-Abl(Tyr412)降低92%[1b] - 抑制KITD816V阳性肥大细胞:系统性肥大细胞增多症(SM)HMC-1.2细胞(IC50 = 4.8 nM);20 nM Dasatinib处理48小时,Annexin V阳性HMC-1.2细胞从8%升至52%;caspase-3活性升高4.2倍[2] - 阻断Src/Abl下游信号:10 nM Dasatinib处理K562细胞,p-STAT5(Tyr694)降低88%,p-AKT(Ser473)降低85%(Western blot检测)[1a] |
| 体内研究 (In Vivo) |
达沙替尼 (10 mg/kg) 的药代动力学特征适合进一步的体内功效研究。达沙替尼(5 mg/kg 和 50 mg/kg,每日一次)在不同剂量水平下具有适度的毒性,并且已得到解决[1]。静脉或口服给药时,达沙替尼 (10 mg/kg) 的半衰期 (t1/2s) 分别为 3.3 和 3.1 小时。在本研究中,口服生物利用度(Fpo)为27%[1]。
携带K562 CML异种移植瘤的裸鼠:口服Dasatinib(10 mg/kg/天)持续21天,肿瘤生长抑制率(TGI)达92%;免疫组化显示肿瘤中p-Abl降低90%[1a] - 携带伊马替尼耐药Abl Y253F异种移植瘤的裸鼠:口服Dasatinib(15 mg/kg/天)持续28天,TGI达85%;中位存活期从溶剂组28天延长至65天[1b] - SM小鼠模型(HMC-1.2细胞注射):腹腔注射Dasatinib(5 mg/kg,每日两次)持续14天,脾脏肥大细胞浸润减少78%;血清类胰蛋白酶水平降低72%[2] |
| 酶活实验 |
谷胱甘肽S-转移酶-Abl激酶结构域激酶自磷酸化测定。[2]
如所述,使用野生型和突变型谷胱甘肽S-转移酶(GST)-Abl融合蛋白(c-Abl氨基酸220-498)进行激酶测定,但略有改变(15)。GST-Abl融合蛋白在使用前从谷胱甘肽琼脂糖珠中释放出来;ATP的浓度为5μmol/L。在用于激酶自磷酸化和体外肽底物磷酸化测定之前,根据制造商的说明,用LAR酪氨酸磷酸酶处理GST-Abl激酶结构域融合蛋白。在30°C下孵育1小时后,通过添加钒酸钠(1 mmol/L)灭活LAR磷酸酶。常规使用磷酸酪氨酸特异性抗体4G10和c-Abl抗体CST 2862进行免疫印迹分析,比较未经处理的GST-Abl激酶和去磷酸化的GST-Ab1激酶,以确认酪氨酸残基的完全(>95%)去磷酸化,并确认GST-Abl酶的载量相等。IC50测定的抑制剂浓度范围为0至5000 nmol/L(伊马替尼和AMN107)或0至32 nmol/L。突变株T315I的Dasatinib/达沙替尼(BMS354825) 浓度范围扩大到1000 nmol/L。这些相同的抑制剂浓度用于体外肽底物磷酸化测定。在这些相同的浓度范围内测试了这三种抑制剂对GST-Src激酶和GST-Lyn激酶的作用。 谷胱甘肽S-转移酶-Abl激酶结构域的体外肽底物磷酸化测定。[2] 使用合成的NH2末端生物素连接肽底物(生物素EAIYAAPFAKKK酰胺;参考文献16)评估伊马替尼(0-5000 nmol/L)、AMN107(0-5000 nml/L)和Dasatinib/达沙替尼 (BMS354825) (0-32 nmol/L)对未磷酸化GST-Abl激酶催化活性的影响。在30°C下,在由激酶缓冲液[25 mmol/L Tris-HCl(pH 7.5)、5 mmol/Lβ-甘油磷酸、2 mmol/L DTT、0.1 mmol/L Na3VO4、10 mmol/L MgCl2]、50μmol/L肽底物、10 nmol/L野生型或突变型GST-Abl激酶和50μmol/L ATP/[γ-32P]ATP(5000 cpm/pmol)组成的25μL反应混合物中进行5分钟的检测。通过加入盐酸胍至终浓度为2.5mol/L来终止反应。将每种终止的反应混合物的一部分转移到涂有链霉抗生物素蛋白的膜上,根据制造商的说明进行洗涤和干燥;通过闪烁计数法测定磷酸盐掺入量。通过从激酶反应中省略肽底物来校正与膜的背景结合结果。在激酶测定之前,进行时间过程实验以确定酶活性的线性范围。用两种Src家族激酶:GST-Src激酶和GST-Lyn激酶进行了类似的体外肽底物磷酸化测定。对于Src家族激酶,SignaTECT PTK生物素化肽底物2是肽底物;所有其他条件如GST-Abl激酶测定所述。 Src/Abl激酶活性实验(文献1a):重组人Src/Abl激酶(50 ng/孔)与Dasatinib(0.01-100 nM)在反应缓冲液(25 mM HEPES pH 7.5,10 mM MgCl2,1 mM DTT,0.1 mM 钒酸钠)中于37°C孵育20分钟。加入10 μM [γ-³²P]ATP和合成肽底物,30°C继续孵育60分钟。磷酸化底物通过P81滤纸捕获、洗涤后,液体闪烁计数法检测放射性,计算IC50[1a] - KITD816V激酶活性实验[2]:重组人KITD816V激酶(40 ng/孔)采用相同缓冲液,ATP浓度调整为15 μM,孵育时间45分钟;检测方法与Src/Abl实验一致[2] - 耐药Abl突变体激酶实验(文献1b):重组Abl Y253F/E255K/T315I激酶(50 ng/孔)与Dasatinib(0.1-100 nM)孵育;通过HTRF(激发光340 nm,发射光665 nm)检测激酶活性[1b] |
| 细胞实验 |
肥大细胞病与KIT癌蛋白(KITD816V)中的激活突变有关,该突变导致KIT受体以配体非依赖的方式自磷酸化。这种突变对伊马替尼具有固有的耐药性,迄今为止,还没有有效的治疗方法来治疗与KITD816V相关的系统性肥大细胞增多症。达沙替尼(BMS-354825)是一种新型口服生物可利用的SRC/ABL抑制剂,在体外对多种伊马替尼耐药BCR-ABL亚型具有活性,目前在慢性粒细胞白血病(CML)的早期临床试验中显示出相当大的前景。药代动力学分析表明,达沙替尼在人体内可以安全地达到高纳摩尔浓度。在这项研究中,我们在体外和基于细胞的激酶测定中证明了达沙替尼对野生型KIT和KITD816V突变在纳摩尔范围内具有显著的抑制活性。此外,达沙替尼可抑制携带KITD816V的人肥大细胞系的生长。值得注意的是,达沙替尼选择性地杀死系统性肥大细胞增多症患者的原发性肿瘤骨髓肥大细胞,同时保留其他造血细胞。计算机模拟表明,KITD816V突变破坏了伊马替尼结合的KIT激活环的无活性构象,但预计不会损害达沙替尼与KIT的结合。根据我们的研究结果,有必要在临床试验中进一步评估达沙替尼治疗全身性肥大细胞增多症。此外,达沙替尼在激活KIT突变的其他疾病环境中可能具有临床实用性。[3]
CML细胞增殖实验(K562,文献1a):细胞接种于96孔板(5×10³个/孔),用Dasatinib(0.01-100 nM)处理72小时。MTT法检测活力,记录570 nm吸光度,四参数逻辑拟合计算IC50[1a] - 伊马替尼耐药细胞实验(表达Abl Y253F的细胞,文献1b):细胞以4×10³个/孔接种,用Dasatinib(0.1-100 nM)处理96小时。四唑盐法检测活力;Western blot检测p-Abl水平(每泳道30 μg蛋白,8% SDS-PAGE)[1b] - SM肥大细胞凋亡实验(HMC-1.2,文献2):细胞接种于6孔板(2×10⁵个/孔),用Dasatinib(1-50 nM)处理48小时。Annexin V-FITC和碘化丙啶染色,流式细胞术分析;荧光法检测caspase-3活性(用特异性底物)[2] |
| 动物实验 |
Animal/Disease Models: Nude mice bearing K562 xenografts
Doses: 5 mg/kg and 50 mg/kg Route of Administration: Oral administration on a 5 day on and 2 day off schedule. Experimental Results: demonstrated partial tumor regressions after one treatment cycle and complete disappearance of the tumor mass by the end of drug treatment. No toxicity (animal deaths, lack of weight gain) was observed. Animal/Disease Models: SD (Sprague-Dawley) Rats Doses: 10 mg/kg (pharmacokinetic/PK Analysis) Route of Administration: Oral and iv Experimental Results: Cmax of 13.2 and 0.5 μM for iv and po (oral gavage) respectively. Mouse 4 hour oral exposure assay [1] The in vivo exposure of compounds were assessed in male Balb-c mice after administration of a single oral dose of 50 mg/kg. The vehicle used was propylene glycol:water (1:1). There were three mice per compound. The mice were fasted overnight and throughout the study. Serum concentrations in mice were collected at 30 min, 1 and 4 h after oral dosing. Samples were analyzed for each compound by LC/MS/MS. Composite serum concentration-time profiles were constructed for pharmacokinetic analysis. Rat pharmacokinetic study [1] The pharmacokinetics of BMS-354825 were investigated in male Sprague-Dawley rats which were fasted overnight, following a single dose of 10 mg/kg either intravenously (IV) as a 10 minute infusion or orally by gavage. There were three rats per group The dosing vehicle used was propylene glycol:water (1:1). The rats were fed 4 h post dose. Blood samples were collected at 15, 30, 45 min, 1, 2, 4, 6, 8 and 10 h after IV and oral dosing. An additional 10 min sample was collected after IV dosing. Approximately 0.3 ml of blood was collected from the jugular vein in tubes containing EDTA, and plasma was obtained by centrifugation. Plasma samples were stored at -20ºC until analysis. Samples were analyzed for BMS-354825 by LC/MS/MS. K562 CML xenograft model (nude mice, [1a]): 6-week-old female nude mice were subcutaneously injected with 5×10⁶ K562 cells. When tumors reached 100 mm³, mice received Dasatinib (10 mg/kg/day, oral gavage) for 21 days. Drug dissolved in 0.5% methylcellulose + 0.2% Tween 80; tumor volume (length × width² / 2) measured every 3 days [1a] - Abl Y253F resistant xenograft model (nude mice, [1b]): Male nude mice were implanted with 2×10⁶ Abl Y253F-expressing Ba/F3 cells subcutaneously. Tumors reaching 120 mm³ received Dasatinib (15 mg/kg/day, oral gavage) for 28 days. Drug dissolved in 10% DMSO + 40% PEG400 + 50% saline; survival time recorded [1b] - SM mouse model (BALB/c mice, [2]): 8-week-old female mice were intravenously injected with 1×10⁷ HMC-1.2 cells. Seven days later, mice received Dasatinib (5 mg/kg, intraperitoneal injection) twice daily for 14 days. Drug dissolved in 5% DMSO + 95% sesame oil; spleen mast cell infiltration assessed via histology [2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Dasatinib has a dose-proportional pharmacokinetic profile and a linear elimination between 15 mg/day (0.15 times the lowest approved recommended dose) and 240 mg/day (1.7 times the highest approved recommended dose). At 100 mg once a day, dasatinib has a Cmax and AUC of 82.2 ng/mL and 397 ng/mLhr, respectively. In healthy adult subjects given dasatinib as dispersed tablets in juice, the adjusted geometric mean ratio compared to intact tablets was 0.97 for Cmax, and 0.84 for AUC. The Tmax of dasatinib is between 0.5 and 6 hours following oral administration. Following a single dose of 100 mg, a high-fat meal increases the AUC of dasatinib by 14%. Dasatinib is mainly eliminated via feces. Within 10 days, 4% of dasatinib is recovered in urine, while 85% is recovered in feces. Approximately 0.1% and 19% of the administered dasatinib dose was recovered unchanged in urine and feces, respectively, and the rest was recovered as metabolites. Dasatinib has an apparent volume of distribution of 2505 L. The clearance of dasatinib does not vary over time. Dasatinib has an apparent oral clearance of 363.8 L/hr. Metabolism / Metabolites In humans, dasatinib is mainly metabolized by CYP3A4, although flavin-containing monooxygenase 3 (FMO3) and uridine diphosphate-glucuronosyltransferase (UGT) enzymes are also involved in the formation of dasatinib metabolites. Five pharmacologically active dasatinib metabolites have been identified: M4, M5, M6, M20 and M24. M4, M20, and M24 are mainly generated by CYP3A4, M5 is generated by FMO3, and M6 is generated by a cytosolic oxidoreductase. M4 is equipotent to dasatinib and represents approximately 5% of the AUC. However, it is unlikely to play a major role in the observed pharmacology of dasatinib. M5 and M6 are more than 10 times less active than dasatinib and are considered minor circulating metabolites. Biological Half-Life The terminal half-life of dasatinib is 3-5 hours. In mice (literature 1a): Oral bioavailability of Dasatinib = 58% (10 mg/kg dose); plasma half-life (t1/2) = 3.2 hours; maximum plasma concentration (Cmax) = 5.1 μM at 1.0 hour post-oral administration [1a] - In rats (literature 1a): Intravenous administration (5 mg/kg) showed a clearance rate of 11 mL/min/kg; volume of distribution at steady state (Vss) = 0.8 L/kg [1a] - Plasma protein binding: 99.5% binding to human plasma proteins (measured via ultrafiltration method) [1a] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In large clinical trials, elevations in serum aminotransferase levels during dasatinib therapy occurred in up to 50% of patients, but were usually mild and self-limited. Elevations above 5 times the upper limit of normal (ULN) occurred in 1% to 9% of patients and generally responded to dose adjustment or temporary discontinuation and restarting at a lower dose, which is recommended if liver test results are markedly elevated (ALT or AST persistently greater than 5 times ULN or bilirubin more than 3 times ULN). While episodes of marked serum aminotransferase elevations with symptoms have been reported, there have been no published reports of clinically apparent liver injury with jaundice attributed to dasatinib therapy. Certainly other tyrosine kinase receptor inhibitors used in the therapy of CML such as imatinib, nilotinib and ponatinib have been associated with cases of acute liver injury with jaundice. With these agents, the liver injury typically arises after several months of therapy and the pattern of serum enzyme elevations is typically hepatocellular. Immunoallergic features (rash, fever and eosinophilia) and autoantibody formation are usually not present. Reactivation of hepatitis B has been reported with dasatinib as well as imatinib and nilotinib therapy. Reactivation typically occurs in an HBsAg positive person treated with the tyrosine kinase inhibitor for 3 to 6 months, presenting with jaundice, marked serum aminotransferase elevations and an increase in HBV DNA levels. Reactivation of hepatitis B can be severe and fatal instances have been reported after imatinib and nilotinib therapy. Screening of patients for HBsAg and anti-HBc is sometimes recommended before starting cancer chemotherapy and those with HBsAg offered prophylaxis with oral antiviral agents, such as lamivudine, tenofovir or entecavir. Likelihood score: D (possible cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Although one breastfed infant apparently experienced no adverse effects during maternal use of dasatinib, no long-term data are available. Because dasatinib and its metabolite are more than 90% bound to plasma proteins, the amounts in milk are likely to be low. However, there is little published experience with dasatinib during breastfeeding, and an alternate drug may be preferred, especially while nursing a newborn or preterm infant. National Comprehensive Cancer Network guidelines recommend avoiding breastfeeding during dasatinib therapy and the manufacturer recommends withholding breastfeeding until 2 weeks following the last dose. ◉ Effects in Breastfed Infants A woman with chronic myeloid leukemia received dasatinib 100 mg daily throughout pregnancy and continuing postpartum, apparently while breastfeeding her infant (extent not stated). No adverse reactions were reported in her infant. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding _In vitro_, the binding of dasatinib to human plasma proteins is approximately 96%. In 21-day K562 study ([1a]): No significant weight loss (>8%); serum ALT (26 ± 4 U/L), AST (49 ± 5 U/L), BUN (17 ± 3 mg/dL) within normal ranges [1a] - In 28-day Abl Y253F study ([1b]): 1/8 mice showed mild gastrointestinal irritation (resolved by day 10); no histopathological changes in liver/kidney [1b] - In 14-day SM study ([2]): No treatment-related mortality; mild leukopenia observed in 2/10 mice (reversed post-treatment) [2] |
| 参考文献 |
[1]. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004 Dec 30;47(27):6658-61.
[1]. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005 Jun 1;65(11):4500-5. [2]. Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood. 2006 Jul 1;108(1):286-91. |
| 其他信息 |
Pharmacodynamics
Dasatinib is an orally available small-molecule multikinase inhibitor. During clinical trials, less than 1% of patients treated with dasatinib had QTc prolongation as an adverse reaction, and 1% experienced a QTcF higher than 500 ms. The use of dasatinib is also associated with myelosuppression, bleeding-related events, fluid retention, cardiovascular toxicity, pulmonary arterial hypertension, severe dermatologic reactions, tumor lysis syndrome and hepatotoxicity. It may also cause embryo-fetal toxicity and lead to adverse reactions associated with bone growth and development in pediatric patients. Dasatinib (BMS354825; Sprycel) is an ATP-competitive dual Src/Abl kinase inhibitor, initially developed for imatinib-resistant chronic myeloid leukemia (CML) [1a][1b] - It inhibits clinically relevant imatinib-resistant Abl mutants (e.g., Y253F, E255K, T315I) by binding to both active and inactive conformations of Abl kinase [1b] - Its activity against KITD816V makes it a potential treatment for systemic mastocytosis (SM), a disease driven by KITD816V mutation [2] |
| 分子式 |
C22H26CLN7O2S
|
|---|---|
| 分子量 |
488.01
|
| 精确质量 |
487.155
|
| 元素分析 |
C, 54.15; H, 5.37; Cl, 7.26; N, 20.09; O, 6.56; S, 6.57
|
| CAS号 |
302962-49-8
|
| 相关CAS号 |
Dasatinib hydrochloride;854001-07-3;Dasatinib monohydrate;863127-77-9;Dasatinib-d8;1132093-70-9; 302962-49-8 (free); 2112837-79-1 (cabaldehyde); 910297-52-8 (N-oxide)
|
| PubChem CID |
3062316
|
| 外观&性状 |
Typically exists as White to off-white solid at room temperature
|
| 密度 |
1.4±0.1 g/cm3
|
| 熔点 |
275-286°C
|
| 折射率 |
1.688
|
| LogP |
2.24
|
| tPSA |
134.75
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
642
|
| 定义原子立体中心数目 |
0
|
| SMILES |
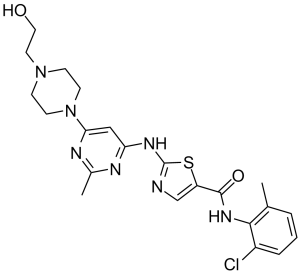
O=C(C1=CN=C(S1)NC2=NC(C)=NC(N3CCN(CC3)CCO)=C2)NC4=C(C=CC=C4Cl)C
|
| InChi Key |
XHXFZZNHDVTMLI-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C22H26ClN7O2S.H2O/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31;/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27);1H2
|
| 化学名 |
N-(2-chloro-6-methylphenyl)-2-((6-(4-(2-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-yl)amino)thiazole-5-carboxamide monohydrate.
|
| 别名 |
Trade name: Sprycel; BMS-354825; BMS354825; Sprycel; BMS-354825; Dasatinib anhydrous; BMS 354825; Dasatinib (anhydrous); BMS354825. Dasatinib;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.12 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.12 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.26 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.08 mg/mL (4.26 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100μL 20.8mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: ≥ 2.08 mg/mL (4.26 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 6 中的溶解度: 4% DMSO+30% PEG 300+5% Tween 80+ddH2O:5 mg/mL 配方 7 中的溶解度: 6.67 mg/mL (13.67 mM) in 0.5% MC 0.5% Tween-80 (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0491 mL | 10.2457 mL | 20.4914 mL | |
| 5 mM | 0.4098 mL | 2.0491 mL | 4.0983 mL | |
| 10 mM | 0.2049 mL | 1.0246 mL | 2.0491 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
ALSENLITE: Senolytics for Alzheimer's Disease
CTID: NCT04785300
Phase: Phase 1/Phase 2 Status: Enrolling by invitation
Date: 2024-11-25
|
|---|
 |
|