| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| 100g | |||
| Other Sizes |
| 体外研究 (In Vitro) |
双氯芬酸的 IC50 为 7±3 nM,可有效破坏 U937 细胞中 COX-1 介导的微粒体形成[1]。双氯芬酸(1-60 μM;1 天)以浓度依赖性方式杀死神经干细胞 (NSC)。每六天,双氯芬酸 (10–60 μM;1) 就会增加 caspase-3 的表达。
|
|---|---|
| 体内研究 (In Vivo) |
松鼠猴每天两次服用 1 mg/kg,持续 4 天,在用双氯芬酸(3 mg/kg,bid)治疗 5 天后,粪便 Cr 排泄也显着增加 [1]。用双氯芬酸(10 mg/kg;在触发因子通过药物途径传递之前给药)治疗的 Wistar 大鼠显示出抗炎作用 [1]。
|
| 细胞实验 |
细胞活力测定[3]
细胞类型:神经干细胞 (NSC) 测试浓度:1、3、10、30、60 μM 孵育时间: 1 天 实验结果: 细胞死亡的诱导具有浓度依赖性,浓度高达 60 μM 时效果不饱和。 蛋白质印迹分析[3] 细胞类型:神经干细胞 (NSC) 测试浓度:10、30 或 60 μM 孵育持续时间:6小时 实验结果: caspase-3的激活以浓度依赖性方式增加。 |
| 动物实验 |
Animal/Disease Models: Male SD (SD (Sprague-Dawley)) rats (150±200 g) [1]
Doses: 3 mg/kg Route of Administration: Oral administration, bid, for 5 days Experimental Results: Caused a significant increase in fecal 51Cr excretion. Animal/Disease Models: Wistar rat (150-175 g) formalin-induced rat paw edema model [2] Doses: 10 mg/kg Route of Administration: By oral route before inducing inflammation Experimental Results: Shown in vivo Anti-inflammatory activity (% edema inhibition = 29.2 at 1 hour; 22.2 at 3 hrs (hrs (hours)); 20 at 6 hrs (hrs (hours))). |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Diclofenac is completely absorbed from the GI tract but likely undergoes significant first pass metabolism with only 60% of the drug reaching systemic circulation unchanged. Many topical formulations are absorbed percutaneous and produce clinically significant plasma concentrations. Absorption is dose proportional over the range of 25-150 mg. Tmax varies between formulations with the oral solution reaching peak plasma concentrations in 10-40min, the enteric coated tablet in 1.5-2h, and the sustained- and extended-release formulations prolonging Tmax even further. Administration with food has no significant effects on AUC but does delay Tmax to 2.5-12h. Diclofenac is mainly eliminated via metabolism. Of the total dose, 60-70% is eliminated in the urine and 30% is eliminated in the feces. No significant enterohepatic recycling occurs. Diclofenac has a total volume of distribution of 5-10 L or 0.1-0.2 L/kg. The volume of the central compartment is 0.04 L/kg. Diclofenac distributes to the synovial fluid reaching peak concentration 2-4h after administration. There is limited crossing of the blood brain barrier and cerebrospinal fluid concentrations only reach 8.22% of plasma concentrations. Doses of 50 mg delivered via intramuscular injection produced no detectable diclofenac concentrations in breast milk, however metabolite concentrations were not investigated. Diclofenac has been shown to cross the placenta in mice and rats but human data is unavailable. Diclofenac has a plasma clearance 16 L/h. Onset of absorption is delayed when diclofenac sodium is administered orally as delayed-release (enteric-coated) tablets, but the extent of absorption does not appear to be affected. /Diclofenac sodium/ Measurable plasma concentrations of diclofenac have been observed in some fasting individuals within 10 minutes of receiving diclofenac potassium conventional tablets. /Diclofenac potassium/ Diclofenac sodium and diclofenac potassium are almost completely absorbed from the GI tract; however, the drugs undergo extensive first-pass metabolism in the liver, with only about 50-60% of a dose of diclofenac sodium or diclofenac potassium reaching systemic circulation as unchanged drug. Diclofenac also is absorbed into systemic circulation following rectal administration and percutaneously following topical application to the skin as a gel or transdermal system. Food decreases the rate of absorption of conventional tablets of diclofenac potassium and of delayed-release (enteric-coated) tablets of diclofenac sodium, resulting in delayed and decreased peak plasma concentrations; however, the extent of absorption is not affected substantially. When diclofenac potassium conventional tablets are administered with food, time to achieve peak plasma concentrations of the drug is increased and peak plasma concentrations of the drug are decreased by approximately 30%. When single doses of diclofenac sodium delayed-release (enteric-coated) tablets are taken with food, the onset of absorption usually is delayed by 1-4.5 hours but may be delayed up to 12 hours in some patients. These food-induced alterations in GI absorption of the drug result from delayed transit of the delayed-release (enteric-coated) tablets to the small intestine, the site of dissolution. When diclofenac sodium extended-release tablets are taken with food, onset of absorption is delayed 1-2 hours and peak plasma concentrations are increased two-fold; however, extent of absorption is not substantially affected. Absorption of diclofenac does not appear to be affected substantially by the presence of food following continuous dosing of the drug. Antacids also may decrease the rate but not the extent of absorption of diclofenac. For more Absorption, Distribution and Excretion (Complete) data for DICLOFENAC (11 total), please visit the HSDB record page. Metabolism / Metabolites Diclofenac undergoes oxidative metabolism to hydroxy metabolites as well as conjugation to glucuronic acid, sulfate, and taurine. The primary metabolite is 4'-hydroxy diclofenac which is generated by CYP2C9. This metabolite is very weakly active with one thirtieth the activity of diclofenac. Other metabolites include 3'-hydroxy diclofenac, 3'-hydroxy-4'methoxy diclofenac, 4',5-dihydroxy diclofenac, an acylglucuronide conjugate, and other conjugate metabolites. The extent of metabolism of diclofenac sodium in excised viable human skin was investigated using combination HPLC and radioactivity assay. In an earlier diffusion experiment using an in vitro flow-through diffusion system, radiolabelled diclofenac sodium in either lotion (Pennsaid) or aqueous solution was applied to viable human skin, either as single dose or multiple dose (8 times over 2 days). In this study, the receptor fluid samples from the diffusion experiment were subjected to extraction and the aliquot was analysed using HPLC to separate diclofenac and authentic metabolites. Based on the radioactivity of each HPLC fraction, the collection time of the fractions was compared with the retention time of diclofenac and metabolites in standard solutions. The samples from a single or multiple dose application of lotion showed radioactivity in mainly one fraction, whose retention time corresponded with diclofenac. Other HPLC fractions showed none or only small amounts of radioactivity within the error range of the assay. The same results were obtained with the pooled samples from the application of the lotion or of aqueous solution. The results suggest that diclofenac sodium does not undergo metabolism in viable human epidermis during percutaneous absorption in vitro. Hence, with topical application to human skin in vivo, diclofenac will be delivered with minimal, if any, metabolism. /Diclofenac sodium/ In humans, metabolism of the commonly used nonsteroidal antiinflammatory drug diclofenac /compound/ 1 yields principally the 4'-hydroxy /compound/ 2, 5-hydroxy /compound/ 3, and acyl glucuronide /compound/ 4 metabolites. All three metabolites have been implicated in rare idiosyncratic adverse reactions associated with this widely used drug. Therefore, for mechanistic toxicological studies of /compound/ 1, substantial quantities of 2-4 are required and their syntheses and characterization are described here. Key steps were a convenient two-step preparation of aniline /compound/ 5 from phenol, efficient and selective 6-iodination of amide /compound/ 18, and high-yielding Ullmann couplings to generate diarylamines /compound/ 11 and /compound/ 21. The acyl glucuronide /compound/ 4 was obtained by Mitsunobu reaction of /compound/ 1 (free acid) with allyl glucuronate /compound/ 23 followed by Pd(0) deprotection, using a modification of a published procedure. /Investigators/ report full characterization of /compound/ 4 ... /Investigators/ report also the metabolic fates of the synthetic metabolites: /compound/ 2 and /compound/ 3 were glucuronidated in rats, but only /compound/ 3 formed glutathione adducts in vivo and by enzymatic synthesis via a quinoneimine intermediate. A previously undescribed glutathione adduct of /compound/ 3 was obtained by enzymatic synthesis. Compound /compound/ 4 formed an imine-linked protein conjugate as evinced by sodium cyanoborohydride trapping. Diclofenac is eliminated predominantly (approximately 50%) as its 4'-hydroxylated metabolite in humans, whereas the acyl glucuronide (AG) pathway appears more important in rats (approximately 50%) and dogs (>80-90%). However, previous studies of diclofenac oxidative metabolism in human liver microsomes (HLMs) have yielded pronounced underprediction of human in vivo clearance. We determined the relative quantitative importance of 4'-hydroxy and AG pathways of diclofenac metabolism in rat, dog, and human liver microsomes. Microsomal intrinsic clearance values (CL(int) = V(max)/K(m)) were determined and used to extrapolate the in vivo blood clearance of diclofenac in these species. Clearance of diclofenac was accurately predicted from microsomal data only when both the AG and the 4'-hydroxy pathways were considered. However, the fact that the AG pathway in HLMs accounted for ~75% of the estimated hepatic CL(int) of diclofenac is apparently inconsistent with the 4'-hydroxy diclofenac excretion data in humans. Interestingly, upon incubation with HLMs, significant oxidative metabolism of diclofenac AG, directly to 4'-hydroxy diclofenac AG, was observed. The estimated hepatic CL(int) of this pathway suggested that a significant fraction of the intrahepatically formed diclofenac AG may be converted to its 4'-hydroxy derivative in vivo. Further experiments indicated that this novel oxidative reaction was catalyzed by CYP2C8, as opposed to CYP2C9-catalyzed 4'-hydroxylation of diclofenac. These findings may have general implications in the use of total (free + conjugated) oxidative metabolite excretion for determining primary routes of drug clearance and may question the utility of diclofenac as a probe for phenotyping human CYP2C9 activity in vivo via measurement of its pharmacokinetics and total 4'-hydroxy diclofenac urinary excretion. The metabolism of (14)C-diclofenac in mice was investigated following a single oral dose of 10 mg/kg. The majority of the drug-related material was excreted in the urine within 24 hr of administration (49.7%). Liquid chromatographic analysis of urine and fecal extracts revealed extensive metabolism to at least 37 components, with little unchanged diclofenac excreted. Metabolites were identified using a hybrid linear ion-trap mass spectrometer via exact mass determinations of molecular ions and subsequent multi-stage fragmentation. The major routes of metabolism identified included: 1) conjugation with taurine; and 2) hydroxylation (probably at the 4'-and 5-arene positions) followed by conjugation to taurine, glucuronic acid or glucose. Ether, rather than acyl glucuronidation, predominated. There was no evidence for p-benzoquinone-imine formation (i.e. no glutathione or mercapturic acid conjugates were detected). A myriad of novel minor drug-related metabolites were also detected, including ribose, glucose, sulfate and glucuronide ether-linked conjugates of hydroxylated diclofenac derivatives. Combinations of these hydroxylated derivatives with acyl conjugates (glucose, glucuronide and taurine) or N-linked sulfation or glucosidation were also observed. Acyl- or amide-linked-conjugates of benzoic acid metabolites and several indolinone derivatives with further hydroxylated and conjugated moieties were also evident. The mechanisms involved in the generation of benzoic acid and indolinone products indicate the formation reactive intermediates in vivo that may possibly contribute to hepatotoxicity. For more Metabolism/Metabolites (Complete) data for DICLOFENAC (7 total), please visit the HSDB record page. Diclofenac has known human metabolites that include 4'-hydroxydiclofenac, 5-hydroxydiclofenac, and (2S,3S,4S,5R)-6-[2-[2-(2,6-Dichloroanilino)phenyl]acetyl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid. Diclofenac is a known human metabolite of aceclofenac. Hepatic. Route of Elimination: Diclofenac is eliminated through metabolism and subsequent urinary and biliary excretion of the glucuronide and the sulfate conjugates of the metabolites. Little or no free unchanged diclofenac is excreted in the urine. Approximately 65% of the dose is excreted in the urine and approximately 35% in the bile as conjugates of unchanged diclofenac plus metabolites. Half Life: 2 hours Biological Half-Life The terminal half-life of diclofenac is approximately 2 h, however the apparent half-life including all metabolites is 25.8-33 h. Following application of diclofenac epolamine transdermal system, the elimination half-life of diclofenac is approximately 12 hours. /Diclofenac epolamine/ Following IV administration of diclofenac sodium in healthy adults, the half-life of diclofenac reportedly averages about 3 minutes in the initial distribution phase, about 16 minutes in the intermediate (redistribution) phase, and about 1-2 hours in the terminal (elimination) phase. /Diclofenac sodium/ Elimination: Up to 6 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Elevated serum aminotransferase levels have been reported in up to 15% of patients taking oral diclofenac chronically, but are greater than 3 times the upper limit of normal in only 2% to 4% (Cases 1 and 2). Clinically apparent and symptomatic liver disease with jaundice due to diclofenac is rare (1 to 5 cases per 100,000 prescriptions, occurring in 1 to 5 persons per 10,000 exposed). Nevertheless, more than a hundred instances of clinically apparent liver injury due to diclofenac have been reported in the literature and, in most case series, diclofenac ranks in the top 10 causes of drug induced liver injury. The time to onset of liver injury varies from within a week to over a year after starting. The majority of cases present within 2 to 6 months (Cases 3 and 4), and the more severe cases tend to present earlier. The pattern of injury is almost exclusively hepatocellular, although cases presenting with mixed patterns have been reported. The clinical picture is that of jaundice preceded by anorexia, nausea, vomiting and malaise. Fever and rash occur in 25% of cases and some cases have immunoallergic features, while others resemble chronic hepatitis and have autoimmune features. In most cases, liver histology reveals an acute lobular hepatitis. However, a cases with prolonged latency diclofenac hepatotoxicity can have clinical and histologic features of chronic hepatitis (Case 2). There seems to be greater susceptibility for diclofenac liver injury among women than men. The injury can be severe, and several cases of acute liver failure have been attributed to diclofenac. Likelihood score: A (well known cause of clinically apparent liver injury). Topical forms of diclofenac (solutions, gels, creams, patches) have been associated with only a low rate of serum enzyme elevations (generally less than 1%) that may be no greater than occurs with placebo or vehicle application. However, product labels for topical diclofenac mention the possibility of liver injury and at least one case of clinically apparent liver injury attributed to topical diclofenac has been reported in the literature. Nevertheless, clinically apparent liver injury due to topical forms of diclofenac must be exceedingly rare. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Data on excretion of diclofenac into milk are poor, but the drug has a short half-life and little glucuronide metabolite formation. Levels in milk appear to be quite low. Most reviewers consider diclofenac to be acceptable during breastfeeding. Other agents having more published information may be preferred, especially while nursing a newborn or preterm infant. Maternal use of diclofenac topical gel or eye drops would not be expected to cause any adverse effects in breastfed infants. To substantially diminish the amount of drug that reaches the breastmilk after using eye drops, place pressure over the tear duct by the corner of the eye for 1 minute or more, then remove the excess solution with an absorbent tissue. ◉ Effects in Breastfed Infants In one study, 30 mothers undergoing elective cesarean section were allowed to use 25 mg diclofenac suppositories along with either spinal or spinal and epidural anesthesia with a local anesthetic after delivery. The spinal anesthetic group used an average of 56 mg of diclofenac on the day of delivery and 33 mg on the next day whereas the women receiving both spinal and epidural anesthesia used 21 and 18 mg. No mention was made of adverse effects on the breastfed infants. A breastfed infant developed urticaria on day 15 of life. Her mother had been taking diclofenac (dosage unspecified) for pain since her cesarean section delivery. Diclofenac is a possible cause of the urticaria; however, the infant had also received hepatitis B vaccination 7 days before and the authors thought that it was a more likely cause of the reaction. ◉ Effects on Lactation and Breastmilk A randomized, double-blind study was performed in pregnant women scheduled for cesarean section under spinal anesthesia with bupivacaine and fentanyl. Patients received either 100 mg diclofenac (n = 100), 100 mg tramadol (n = 100) or placebo (glycerin suppositories) n = 100, all given as rectal suppositories every 8 hours for the first 24 hours after surgery. The time to initiate breastfeeding was significantly shorter among mothers who received diclofenac than a placebo, 1.5 vs 4.1 hours with breastfeeding support and 3.5 vs 6.2 hours without support. Diclofenac was slightly more effective than tramadol among mothers who received no support (3.5 vs 3.7 hours). Protein Binding Diclofenac is over 99.7% bound to serum proteins, primarily albumin. It is undergoes limited binding to lipoproteins as well with 1.1% bound to HDL, 0.3% to LDL, and 0.15% to VLDL. |
| 参考文献 |
|
| 其他信息 |
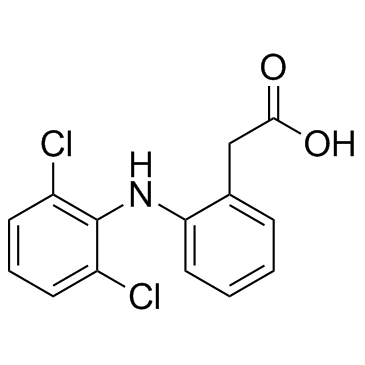
Diclofenac is a monocarboxylic acid consisting of phenylacetic acid having a (2,6-dichlorophenyl)amino group at the 2-position. It has a role as a non-narcotic analgesic, an antipyretic, an EC 1.14.99.1 (prostaglandin-endoperoxide synthase) inhibitor, a xenobiotic, an environmental contaminant, a drug allergen and a non-steroidal anti-inflammatory drug. It is a secondary amino compound, an amino acid, a dichlorobenzene, an aromatic amine and a monocarboxylic acid. It is functionally related to a phenylacetic acid and a diphenylamine. It is a conjugate acid of a diclofenac(1-).
Diclofenac is a phenylacetic acid derivative and non-steroidal anti-inflammatory drug (NSAID). NSAIDs inhibit cyclooxygenase (COX)-1 and-2 which are the enzyme responsible for producing prostaglandins (PGs). PGs contribute to inflammation and pain signalling. Diclofenac, like other NSAIDs, is often used as first line therapy for acute and chronic pain and inflammation from a variety of causes. Diclofenac was the product of rational drug design based on the structures of [phenylbutazone], [mefenamic acid], and [indomethacin]. The addition of two chlorine groups in the ortho position of the phenyl ring locks the ring in maximal torsion which appears to be related to increased potency. It is often used in combination with [misoprostol] to prevent NSAID-induced gastric ulcers. Diclofenac was first approved by the FDA in July 1988 under the trade name Voltaren, marketed by Novartis (previously Ciba-Geigy). Diclofenac is a Nonsteroidal Anti-inflammatory Drug. The mechanism of action of diclofenac is as a Cyclooxygenase Inhibitor. The physiologic effect of diclofenac is by means of Decreased Prostaglandin Production. Diclofenac is a commonly used nonsteroidal antiinflammatory drug (NSAID) used for the therapy of chronic forms of arthritis and mild-to-moderate acute pain. Therapy with diclofenac in full doses is frequently associated with mild serum aminotransferase elevations and, in rare instances, can lead to serious clinically apparent, acute or chronic liver disease. Diclofenac is a nonsteroidal benzeneacetic acid derivative with anti-inflammatory activity. As a nonsteroidal anti-inflammatory drug (NSAID), diclofenac binds and chelates both isoforms of cyclooxygenase (COX-1 and-2), thereby blocking the conversion of arachidonic acid to pro-inflammatory-proprostaglandins. This agent also may inhibit COX-2-mediated tumor angiogenesis. When inhibiting COX-2, diclofenac may be effective in relieving pain and inflammation; when inhibiting COX-1, it may produce unacceptable gastrointestinal side effects. This agent may be more active against COX-2 than several other carboxylic acid-containing NSAIDs. (NCI04) A non-steroidal anti-inflammatory agent (NSAID) with antipyretic and analgesic actions. It is primarily available as the sodium salt. A non-steroidal anti-inflammatory agent (NSAID) with antipyretic and analgesic actions. It is primarily available as the sodium salt. Drug Indication Diclofenac is indicated for use in the treatment of pain and inflammation from varying sources including inflammatory conditions such as osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis, as well as injury-related inflammation due to surgery and physical trauma. It is often used in combination with [misoprostol] as a gastro-protective agent in patients with high risk of developing NSAID-induced ulcers. FDA Label Mechanism of Action Diclofenac inhibits cyclooxygenase-1 and -2, the enzymes responsible for production of prostaglandin (PG) G2 which is the precursor to other PGs. These molecules have broad activity in pain and inflammation and the inhibition of their production is the common mechanism linking each effect of diclofenac. PGE2 is the primary PG involved in modulation of nociception. It mediates peripheral sensitization through a variety of effects. PGE2 activates the Gq-coupled EP1 receptor leading to increased activity of the inositol trisphosphate/phospholipase C pathway. Activation of this pathway releases intracellular stores of calcium which directly reduces action potential threshold and activates protein kinase C (PKC) which contributes to several indirect mechanisms. PGE2 also activates the EP4 receptor, coupled to Gs, which activates the adenylyl cyclase/protein kinase A (AC/PKA) signaling pathway. PKA and PKC both contribute to the potentiation of transient receptor potential cation channel subfamily V member 1 (TRPV1) potentiation, which increases sensitivity to heat stimuli. They also activate tetrodotoxin-resistant sodium channels and inhibit inward potassium currents. PKA further contributes to the activation of the P2X3 purine receptor and sensitization of T-type calcium channels. The activation and sensitization of depolarizing ion channels and inhibition of inward potassium currents serve to reduce the intensity of stimulus necessary to generate action potentials in nociceptive sensory afferents. PGE2 act via EP3 to increase sensitivity to bradykinin and via EP2 to further increase heat sensitivity. Central sensitization occurs in the dorsal horn of the spinal cord and is mediated by the EP2 receptor which couples to Gs. Pre-synaptically, this receptor increases the release of pro-nociceptive neurotransmitters glutamate, CGRP, and substance P. Post-synaptically it increases the activity of AMPA and NMDA receptors and produces inhibition of inhibitory glycinergic neurons. Together these lead to a reduced threshold of activating, allowing low intensity stimuli to generate pain signals. PGI2 is known to play a role via its Gs-coupled IP receptor although the magnitude of its contribution varies. It has been proposed to be of greater importance in painful inflammatory conditions such as arthritis. By limiting sensitization, both peripheral and central, via these pathways NSAIDs can effectively reduce inflammatory pain. PGI2 and PGE2 contribute to acute inflammation via their IP and EP2 receptors. Similarly to β adrenergic receptors these are Gs-coupled and mediate vasodilation through the AC/PKA pathway. PGE2 also contributes by increasing leukocyte adhesion to the endothelium and attracts the cells to the site of injury. PGD2 plays a role in the activation of endothelial cell release of cytokines through its DP1 receptor. PGI2 and PGE2 modulate T-helper cell activation and differentiation through IP, EP2, and EP4 receptors which is believed to be an important activity in the pathology of arthritic conditions. By limiting the production of these PGs at the site of injury, NSAIDs can reduce inflammation. PGE2 can cross the blood-brain barrier and act on excitatory Gq EP3 receptors on thermoregulatory neurons in the hypothalamus. This activation triggers an increase in heat-generation and a reduction in heat-loss to produce a fever. NSAIDs prevent the generation of PGE2 thereby reducing the activity of these neurons. Diclofenac has pharmacologic actions similar to those of other prototypical NSAIAs. The drug exhibits anti-inflammatory, analgesic, and antipyretic activity. The exact mechanisms have not been clearly established, but many of the actions appear to be associated principally with the inhibition of prostaglandin synthesis. Diclofenac inhibits the synthesis of prostaglandins in body tissues by inhibiting cyclooxygenase; at least 2 isoenzymes, cyclooxygenase-1 (COX-1) and -2 (COX-2) (also referred to as prostaglandin G/H synthase-1 (PGHS-10 and -2 (PGHS-2), respectively), have been identified that catalyze the formation of prostaglandins in the arachidonic acid pathway. Diclofenac, like other prototypical NSAIAs, inhibits both COS-1 and COS-2. Although the exact mechanisms have not been clearly established, NSAIAs appear to exert anti-inflammatory, analgesic, and antipyretic activity principally through inhibition of the COS-2 isoenzyme; COX-1 inhibition presumably is responsible for the drugs' unwanted effects on GI mucosa and platelet aggregation. As for all non-steroidal anti-inflammatory drugs the pharmacodynamic effects of diclofenac sodium are of anti-inflammatory, analgesic and antipyretic character due to the decrease of the prostaglandin synthesis from arachidonic acid by inhibition of the cyclo-oxygenase activity. It also induces deleterious effects on gastric and intestinal mucosa and an inhibition of platelet aggregation. /Diclofenac sodium/ |
| 分子式 |
C14H11CL2NO2
|
|---|---|
| 分子量 |
296.15
|
| 精确质量 |
295.016
|
| CAS号 |
15307-86-5
|
| 相关CAS号 |
Diclofenac diethylamine;78213-16-8;Diclofenac-d4;153466-65-0;Diclofenac Sodium;15307-79-6;Diclofenac potassium;15307-81-0;Diclofenac-13C6;1261393-71-8
|
| PubChem CID |
3033
|
| 外观&性状 |
White to light yellow solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
412.0±45.0 °C at 760 mmHg
|
| 熔点 |
156-158ºC
|
| 闪点 |
203.0±28.7 °C
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
| 折射率 |
1.662
|
| LogP |
4.06
|
| tPSA |
49.33
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
19
|
| 分子复杂度/Complexity |
304
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
DCOPUUMXTXDBNB-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19)
|
| 化学名 |
2-[2-(2,6-dichloroanilino)phenyl]acetic acid
|
| 别名 |
Diclofenac acid Dichlofenac Voltarol Voltaren
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~125 mg/mL (~422.08 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (7.02 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (7.02 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3767 mL | 16.8833 mL | 33.7667 mL | |
| 5 mM | 0.6753 mL | 3.3767 mL | 6.7533 mL | |
| 10 mM | 0.3377 mL | 1.6883 mL | 3.3767 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。