| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Multikinase inhibitor with potent activity against Aurora A kinase (Ki = 1.8 nM), Aurora B kinase (IC₅₀ = 45 nM), VEGFR2 (IC₅₀ = 26 nM), FGFR1 (IC₅₀ = 15 nM), and PDGFRβ (IC₅₀ = 22 nM) [1]
- In multiple myeloma (MM) cells, it selectively inhibits Aurora A kinase (EC₅₀ = 5 nM for inhibiting Aurora A-mediated TPX2 phosphorylation) and shows minimal activity against Aurora B (EC₅₀ > 500 nM) [2] |
|---|---|
| 体外研究 (In Vitro) |
ENMD-2076 针对 Aurora A 而非 Aurora B (IC50=350 nM)。 ENMD-2076 抑制 HUVEC 增殖,IC50 为 0.15 mM。 10 种人类白血病细胞系的 IC50 值范围为 0.025 至 0.53 mM。在该图中,MV4:11 细胞的反应性最强,其反应系数超过 4 倍。用 ENMD-2076 处理的淋巴瘤来源的 U937 细胞系显示 G2-M 期停滞和细胞凋亡呈剂量依赖性增加。 ENMD-2076 抑制 THP-1 细胞中细胞 Flt3 配体 (FL) 诱导的 Flt3 自磷酸化,该细胞已被证明表达 FL 响应性野生型 Flt-3 (18),IC50 为 28 nM。 ENMD-2076 抑制 MO7e 细胞中干细胞因子 (SCF) 诱导的 Kit 自磷酸化,IC50 为 40 nM。 ENMD-2076 抑制 VEGFR2/KDR 自磷酸化,IC50 为 7 nM [1]。
对实体瘤细胞系的抗增殖活性[1]: ENMD-2076对实体瘤细胞系表现出广谱抗增殖作用,IC₅₀值范围为18 nM至45 nM: - HCT116(结直肠癌):IC₅₀=22 nM - MCF-7(乳腺癌):IC₅₀=30 nM - A549(肺癌):IC₅₀=45 nM - SK-OV-3(卵巢癌):IC₅₀=18 nM - 诱导实体瘤细胞G2/M期停滞与凋亡[1]: 用ENMD-2076(25 nM)处理HCT116细胞24小时,PI染色显示G2/M期细胞比例从对照组的14%升至60%。用30 nM处理MCF-7细胞48小时,膜联蛋白V阳性凋亡细胞达40%,western blot显示切割型caspase-3增加3.2倍。 - 抗血管生成活性[1]: ENMD-2076(50 nM)在Matrigel管形成实验中抑制人脐静脉内皮细胞(HUVEC)管形成达75%,在Boyden小室实验中减少HUVEC迁移达65%;western blot显示HUVEC中VEGFR2(Tyr1175)磷酸化降低80%。 - 对多发性骨髓瘤细胞的抗增殖活性[2]: 对MM细胞系(MM.1S、RPMI-8226、U266),ENMD-2076的IC₅₀分别为12 nM、18 nM和25 nM(72小时MTT实验),对MM细胞的毒性是正常骨髓基质细胞的5倍(后者IC₅₀=125 nM)。 - 在多发性骨髓瘤中的作用机制[2]: 用ENMD-2076(20 nM)处理MM.1S细胞24小时,western blot显示Aurora A介导的TPX2磷酸化降低70%,G2/M期停滞达55%,凋亡率达35%;同时抑制STAT3(Tyr705)磷酸化60%(MM细胞关键存活通路)。 |
| 体内研究 (In Vivo) |
ENMD-2076 治疗可导致统计学上显着的肿瘤生长或消退抑制,且呈剂量依赖性。此外,由于ENMD-2076对快速生长的肿瘤(如A375黑色素瘤)和缓慢生长的肿瘤(如HT29结肠癌)的抑制作用类似,因此肿瘤生长速率和抗肿瘤功效之间不存在相关性,而这在理论上是有丝分裂激酶抑制剂所预期的。除 A375 模型外,研究尚未发现 ENMD-2076 每日剂量高达 302 mg/kg(相当于 200 mg/kg 游离碱)有任何体重减轻或发病迹象。
实体瘤异种移植模型[1]: - HCT116结直肠癌(裸鼠):口服ENMD-2076(50 mg/kg/日)14天,肿瘤生长抑制率(TGI)达75%。处理组肿瘤体积为190±25 mm³,对照组为760±40 mm³(p<0.001);肿瘤CD31染色(血管生成标志物)降低65%。 - MCF-7乳腺癌(裸鼠):口服ENMD-2076(60 mg/kg/日)18天,TGI达70%,肿瘤重量降低40%(处理组0.25±0.03 g,对照组0.62±0.05 g)。 - 多发性骨髓瘤异种移植模型[2]: 携带MM.1S异种移植瘤的SCID小鼠口服ENMD-2076(40 mg/kg/日)21天,肿瘤负荷降低80%(通过人κ轻链ELISA检测),中位生存期延长50%(处理组35天,对照组23天)。免疫组化显示肿瘤中磷酸化TPX2降低75%、磷酸化STAT3降低60%。 |
| 酶活实验 |
Aurora A激酶活性实验(HTRF格式)[1,2]:
- [1] 重组Aurora A(与TPX2结合)与ENMD-2076(0.01–500 nM)、ATP(10 μM)及生物素化TPX2肽在激酶缓冲液(50 mM Tris-HCl、10 mM MgCl₂、1 mM DTT,pH 7.5)中30°C孵育60分钟。EDTA终止反应后,用链霉亲和素-铕穴状化合物和XL665偶联磷酸化抗体检测磷酸化底物,通过竞争性结合模型计算Ki。
- [2] 针对骨髓瘤的实验中,重组Aurora A与ENMD-2076(0.1–100 nM)及组蛋白H3肽孵育,通过FRET信号拟合确定IC₅₀。
- VEGFR2激酶活性实验[1]: 重组VEGFR2与ENMD-2076(0.1–1000 nM)、ATP(20 μM)及生物素化VEGFR2肽在上述缓冲液中37°C孵育45分钟,HTRF法检测磷酸化底物,从剂量-反应曲线计算IC₅₀。 |
| 细胞实验 |
实体瘤细胞增殖与凋亡实验[1]:
- 增殖实验:细胞(2×10³个/孔,96孔板)用ENMD-2076(1–200 nM)处理72小时,MTT法检测吸光度(570 nm),IC₅₀定义为抑制50%活力的浓度。
- 凋亡实验:MCF-7细胞(5×10⁵个/孔)用30 nM ENMD-2076处理48小时,膜联蛋白V-FITC/PI染色后流式细胞术分析。
- 细胞周期实验:HCT116细胞用25 nM处理24小时,乙醇固定后PI/RNase染色,流式细胞术分析周期分布。
- HUVEC管形成实验[1]: 96孔板包被Matrigel后接种HUVEC(1×10⁴个/孔)+ENMD-2076(50 nM),37°C孵育6小时,相差显微镜观察管形成,计数完整管数量。抑制率=[1-(处理组/对照组管数)]×100%。 - 多发性骨髓瘤细胞实验[2]: - 增殖实验:MM细胞(3×10³个/孔)用ENMD-2076(1–100 nM)处理72小时,MTT法检测活力。 - Western blot:MM.1S细胞用20 nM处理24小时,RIPA缓冲液裂解,蛋白用抗磷酸化TPX2、抗磷酸化STAT3及抗β-肌动蛋白抗体检测。 |
| 动物实验 |
Dissolved in water or ENMD-2076 free base in CMC-Tween vehicle (0.075% carboxymethylcellulose, 0.085% Tween 80 in water); 300 mg/kg; Oral gavage Tumor models including HCT-116, HT29, CT-26, A375, MDA-MB-231, H929, OPM-2, MV4;11 and HL60 are established in CB.17 SCID or NCr nude mice.
Solid tumor xenograft models [1]: - HCT116 model: Female nude mice (6–7 weeks) were subcutaneously injected with 5×10⁶ HCT116 cells (PBS/Matrigel 1:1). When tumors reached 100–150 mm³, mice (n=8/group) received oral ENMD-2076 (50 mg/kg, dissolved in 0.5% CMC + 0.1% Tween 80) daily for 14 days. Tumor volume = length×width²/2; weight was measured twice weekly. - MCF-7 model: Mice were implanted with 1×10⁷ MCF-7 cells; ENMD-2076 (60 mg/kg oral) was given daily for 18 days. Tumors were excised for CD31 staining. - Multiple myeloma xenograft model [2]: SCID mice (6–8 weeks) were intravenously injected with 5×10⁶ MM.1S cells. After 7 days, mice (n=6/group) received oral ENMD-2076 (40 mg/kg, dissolved in 0.5% CMC + 0.1% Tween 80) daily for 21 days. Tumor burden was measured via serum human κ-light chain ELISA; survival was monitored for 40 days. |
| 药代性质 (ADME/PK) |
Oral bioavailability [1]:
In male Sprague-Dawley rats, oral ENMD-2076 (20 mg/kg) had 35% bioavailability. Cmax = 1.2 μg/mL at 1.5 h; terminal t₁/₂ = 5.2 h.
- Intravenous pharmacokinetics (rats) [1]: IV ENMD-2076 (5 mg/kg) had CL = 14 mL/min/kg, Vss = 5.0 L/kg, t₁/₂ = 4.8 h. - Plasma protein binding [1]: 96% (human), 95% (rat), 94% (mouse) via equilibrium dialysis (37°C, 4 h, 1 μg/mL drug). - Metabolic stability [1]: In human liver microsomes, t₁/₂ = 4.5 h (moderate stability); major metabolite = monohydroxylated derivative (55% of total, CYP3A4-mediated). |
| 毒性/毒理 (Toxicokinetics/TK) |
Acute oral toxicity (mice) [1]:
Single oral ENMD-2076 up to 2000 mg/kg caused no mortality. Mild activity reduction at ≥1500 mg/kg (recovered in 24 h); no weight loss at ≤1000 mg/kg.
- Chronic toxicity (rats) [1]: Oral ENMD-2076 (50 mg/kg daily, 28 days) caused mild myelosuppression (WBC ↓18%), but normal RBC/platelets and liver/kidney markers (ALT, AST, BUN, creatinine). No organ lesions. - Toxicity in MM model [2]: SCID mice treated with 40 mg/kg ENMD-2076 had no significant weight loss (<5%) or organ toxicity (liver/kidney histopathology normal). |
| 参考文献 |
|
| 其他信息 |
Aurora A Kinase/Tyrosine Kinase Inhibitor ENMD-2076 is an orally bioavailable synthetic small molecule with potential antiangiogenic and antineoplastic activities. Aurora A kinase/tyrosine kinase inhibitor ENMD-2076 selectively binds to and inhibits non-specified tyrosine kinases and Aurora kinases (AKs). The inhibition of AKs may result in the inhibition of cell division and proliferation and may induce apoptosis in tumor cells that overexpress AKs; antiangiogenic activity is related to the inhibition of angiogenic tyrosine kinases. AKs are serine-threonine kinases that play an essential role in mitotic checkpoint control during mitosis and are important regulators of cell division and proliferation.
Mechanism of action [1,2]: - [1] Dual mechanism: (1) Inhibits Aurora A/B (induces mitotic arrest/apoptosis); (2) inhibits VEGFR2/FGFR1 (suppresses angiogenesis). - [2] In MM: Targets Aurora A (disrupts mitosis) and STAT3 (blocks survival signaling), overcoming MM cell dependence on these pathways. - Clinical relevance [1,2]: - [1] Oral activity and antiangiogenic/antiproliferative dual effects make it suitable for solid tumors (colorectal, breast). - [2] Potent against MM cells (including drug-resistant lines) and low normal cell toxicity, supporting use in relapsed/refractory MM. |
| 分子式 |
C21H25N7
|
|---|---|
| 分子量 |
375.470103025436
|
| 精确质量 |
375.217
|
| CAS号 |
934353-76-1
|
| 相关CAS号 |
ENMD-2076 Tartrate;1453868-32-0
|
| PubChem CID |
16041424
|
| 外观&性状 |
White to light yellow solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
535.0±50.0 °C at 760 mmHg
|
| 闪点 |
277.4±30.1 °C
|
| 蒸汽压 |
0.0±1.4 mmHg at 25°C
|
| 折射率 |
1.704
|
| LogP |
1.31
|
| tPSA |
76.2
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
499
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC1=CC(=NN1)NC2=CC(=NC(=N2)/C=C/C3=CC=CC=C3)N4CCN(CC4)C
|
| InChi Key |
BLQYVHBZHAISJM-CMDGGOBGSA-N
|
| InChi Code |
InChI=1S/C21H25N7/c1-16-14-20(26-25-16)23-19-15-21(28-12-10-27(2)11-13-28)24-18(22-19)9-8-17-6-4-3-5-7-17/h3-9,14-15H,10-13H2,1-2H3,(H2,22,23,24,25,26)/b9-8+
|
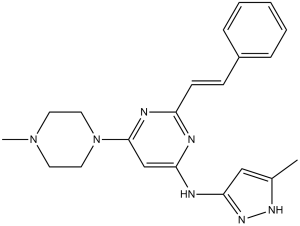
| 化学名 |
(E)-N-(5-methyl-1H-pyrazol-3-yl)-6-(4-methylpiperazin-1-yl)-2-styrylpyrimidin-4-amine.
|
| 别名 |
ENMD 2076; ENMD-2076; ENMD2076.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.66 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (6.66 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.66 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 0.5% CMC+0.25% Tween 80:30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6633 mL | 13.3166 mL | 26.6333 mL | |
| 5 mM | 0.5327 mL | 2.6633 mL | 5.3267 mL | |
| 10 mM | 0.2663 mL | 1.3317 mL | 2.6633 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00806065 | Completed | Drug: ENMD-2076 | Multiple Myeloma | CASI Pharmaceuticals, Inc. | December 2008 | Phase 1 |
| NCT00904787 | Completed | Drug: ENMD-2076 | Relapsed or Refractory Hematological Malignancies |
CASI Pharmaceuticals, Inc. | April 2009 | Phase 1 |
| NCT01104675 | Completed | Drug: ENMD-2076 | Ovarian Cancer Fallopian Cancer |
CASI Pharmaceuticals, Inc. | April 2010 | Phase 2 |
| NCT00658671 | Completed | Drug: ENMD-2076 | Advanced Cancer | CASI Pharmaceuticals, Inc. | April 2008 | Phase 1 |
Antiangiogenic action of ENMD-2076. Mol Cancer Ther. 2011 Jan;10(1):126-37. |
ENMD-2076 inhibits Flt3, VEGFR2/KDR, FGFR-1/2, and Aurora A in vivo. Mol Cancer Ther. 2011 Jan;10(1):126-37. |
ENMD-2076 inhibits blood vessel formation and impacts the growth of established MDA-MB-231 tumors. Mol Cancer Ther. 2011 Jan;10(1):126-37. |