| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|

| 体外研究 (In Vitro) |
埃索美拉唑(25-100 µM;20 小时;MDA-MB-468 细胞)治疗可增强细胞内酸化,进而以剂量依赖性方式抑制体外三阴性乳腺癌细胞的发育 [1]。
|
|---|---|
| 体内研究 (In Vivo) |
通过埃索美拉唑治疗(30-300 mg/kg;口服强饲;每天;持续 19 或 11 天),C57BL/6J 小鼠肺纤维化的进展明显减慢。此外,埃索美拉唑可降低纤维化和炎症的循环指标[2]。
|
| 细胞实验 |
细胞活力测定[1]
细胞类型: MDA-MB-468 细胞 测试浓度: 25 µM、50 µM、75 µM、100 µM 孵育持续时间:20小时 实验结果:在体外以剂量依赖性方式抑制三阴性乳腺癌细胞。 |
| 动物实验 |
Animal/Disease Models: C57BL/6J mice (8 weeks old, 25-30 g) cotton smoke-induced lung injury [2]
Doses: 30 mg/kg, 300 mg/kg Route of Administration: po (oral gavage); daily; continued for 19 Or 11-day Experimental Results: Dramatically inhibited the progression of lung fibrosis in animals. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Omeprazole delayed-release capsules contain an enteric-coated granule formulation of omeprazole (because omeprazole is acid-labile), so that absorption of omeprazole begins only after the granules exit the stomach. Absorption of omeprazole occurs rapidly, with peak plasma concentrations of omeprazole achieved within 0.5-3.5 hours. Absolute bioavailability (compared with intravenous administration) is approximately 30-40% at doses of 20-40 mg, largely due to pre-systemic metabolism. The bioavailability of omeprazole increases slightly upon repeated administration of omeprazole delayed-release capsules. After a single dose oral dose of a buffered solution of omeprazole, negligible (if any) amounts of unchanged drug were excreted in urine. Most of the dose (about 77%) was eliminated in urine as at least six different metabolites. Two metabolites were identified as _hydroxyomeprazole_ and the corresponding _carboxylic acid_. The remainder of the dose was found in the feces. This suggests significant biliary excretion of omeprazole metabolites. Three metabolites have been identified in the plasma, the _sulfide_ and _sulfone_ derivatives of omeprazole, and _hydroxyomeprazole_. These metabolites possess minimal or no antisecretory activity. Approximately 0.3 L/kg, corresponding to the volume of extracellular water. Healthy subject (delayed release capsule), total body clearance 500 - 600 mL/min Geriatric plasma clearance: 250 mL/min Hepatic impairment plasma clearance: 70 mL/min Absorption: rapid. Distribution: Distributed in tissue, particularly gastric parietal cells. Elimination: Renal 72 to 80%. Fecal 18 to 23%. In dialysis - Not readily dialyzable, because of extensive protein binding. To clarify the in vivo first-pass metabolism of omeprazole, the pharmacokinetics were examined after oral, intraduodenal, intraportal venous, and intravenous administration at various doses to rats. Extraction ratios in the liver and intestinal tract were determined from the areas under the concentration-time curve (AUC) for intraportal venous and intravenous administration and from those for intraduodenal and intraportal venous administration, respectively. Assuming that the drug was absorbed from the gastrointestinal tract completely, the hepatic and intestinal extraction ratios were 0.80, 0.63, and 0.59 at doses of 2.5, 5, and 10 mg/kg and 0.70 and 0.73 at doses of 5 and 10 mg/kg, respectively. The bioavailability of orally administered omeprazole was 6.4, 9.6, and 12.6% at the doses of l0, 20, and 40 mg/kg, respectively. There were no differences in the distribution volume of steady state, total clearance, or elimination half-life at any doses. In addition, the AUC value after oral administration (20 mg/kg) in rats acutely intoxicated with CC(14) was 2.4 times larger than that in the control. These findings suggest that omeprazole undergoes a first-pass metabolism in the intestinal mucosa and/or lumen, as well as in the liver, and that the major contribution to the dose-dependent increase in bioavailability is a saturation of the first-pass metabolism in the liver. Omeprazole is distributed into human milk; following oral administration of omeprazole 20 mg in one lactating women, concentrations of the drug were measured in breast milk. For more Absorption, Distribution and Excretion (Complete) data for OMEPRAZOLE (6 total), please visit the HSDB record page. The plasma elimination half-life of esomeprazole is approximately 1 to 1.5 hours. Less than 1% of parent drug is excreted in the urine. Approximately 80% of an oral dose of esomeprazole is excreted as inactive metabolites in the urine, and the remainder is found as inactive metabolites in the feces. Esomeprazole is 97% bound to plasma proteins. Plasma protein binding is constant over the concentration range of 2 to 20 umol/L. The apparent volume of distribution at steady state in healthy volunteers is approximately 16 L. NEXIUM Delayed-Release Capsules and NEXIUM For Delayed-Release Oral Suspension contain a bioequivalent enteric-coated granule formulation of esomeprazole magnesium. Bioequivalency is based on a single dose (40 mg) study in 94 healthy male and female volunteers under fasting condition. After oral administration peak plasma levels (Cmax) occur at approximately 1.5 hours (Tmax). The Cmax increases proportionally when the dose is increased, and there is a three-fold increase in the area under the plasma concentration-time curve (AUC) from 20 to 40 mg. At repeated once-daily dosing with 40 mg, the systemic bioavailability is approximately 90% compared to 64% after a single dose of 40 mg. The mean exposure (AUC) to esomeprazole increases from 4.32 umol*hr/L on Day 1 to 11.2 umol*hr/L on Day 5 after 40 mg once daily dosing. Metabolism / Metabolites Omeprazole is heavily metabolized in the liver by the cytochrome P450 (CYP) enzyme system. The main part of its metabolism depends on the polymorphically expressed CYP2C19, which is responsible for the formation of _hydroxyomeprazole_, the major metabolite found in plasma. The remaining part depends on CYP3A4, responsible for the formation of _omeprazole sulphone_. The in vitro metabolism of omeprazole was studied in human liver microsomes in order to define the metabolic pathways and identify the cytochrome P450 (CYP) isoforms responsible for the formation of the major omeprazole metabolites. 2 The four major metabolites identified in vitro, in tentative order of importance, were hydroxyomeprazole, omeprazole sulphone, 5-O-desmethylomeprazole, and an unidentified compound termed metabolite X. Omeprazole pyridone was also detected but could not be quantitated. Incubation of hydroxyomeprazole and omeprazole sulphone with human microsomes resulted in both cases in formation of the hydroxysulphone. The kinetics of formation of the four primary metabolites studied were biphasic suggesting the involvement of multiple CYP isoforms in each case. Further studies used substrate concentrations at which the high affinity activities predominated. 3 Formation of the major metabolite, hydroxyomeprazole, was significantly correlated with S-mephenytoin hydroxylase and with benzo[a]pyrene metabolism and CYP3A content. Inhibition studies with isoform selective inhibitors also indicated a dominant role of S-mephenytoin hydroxylase with some CYP3A contribution in the formation of hydroxyomeprazole. Correlation and inhibition data for the sulphone and metabolite X were consistent with a predominant role of the CYP3A subfamily in formation of these metabolites. Formation of 5-O-desmethylomeprazole was inhibited by both R, S-mephenytoin and quinidine, indicating that both S-mephenytoin hydroxylase and CYP2D6 may mediate this reaction in human liver microsomes and in vivo. The Vmax/Km (indicator of intrinsic clearance in vivo) for hydroxyomeprazole was four times greater than that for omeprazole sulphone. Consistent with findings in vivo, the results predict that omeprazole clearance in vivo would be reduced in poor metabolisers of mephenytoin due to reduction in the dominant partial metabolic clearance to hydroxyomeprazole. Esomeprazole is extensively metabolized in the liver by the cytochrome P450 (CYP) enzyme system. The metabolites of esomeprazole lack antisecretory activity. The major part of esomeprazole's metabolism is dependent upon the CYP 2C19 isoenzyme, which forms the hydroxy and desmethyl metabolites. The remaining amount is dependent on CYP 3A4 which forms the sulphone metabolite. CYP 2C19 isoenzyme exhibits polymorphism in the metabolism of esomeprazole, since some 3% of Caucasians and 15 to 20% of Asians lack CYP 2C19 and are termed Poor Metabolizers. At steady state, the ratio of AUC in Poor Metabolizers to AUC in the rest of the population (Extensive Metabolizers) is approximately 2. Following administration of equimolar doses, the S- and R-isomers are metabolized differently by the liver, resulting in higher plasma levels of the S- than of the R-isomer. Omeprazole has known human metabolites that include 3-Hydroxyomeprazole, 5-Hydroxyomeprazole, 5'-O-Desmethyl omeprazole, and Omeprazole sulfone. Hepatic. Omeprazole is extensively metabolized by the cytochrome P450 (CYP) enzyme system. The two primary CYP isozymes involved are CYP2C19 and CYP3A4. Metabolism is stereoselective in which the S-isomer is converted to 5'O-desmethylomeprazole via CYP2C19. CYP3A4 converts the S-isomer to 3-hydroxyomeprazole. The R-isomer is converted to 5-hydroxyomeprazole by CYP2C19. CYP3A4 converts the R-isomer to any four different metabolites: 5-hydroxyomeprazole (5-OH OME), omeprazole sulfone (OME sulfone), 5'-O-desmethylomeprazole (5'-desmethyl OME), and 3-hydroxyomeprazole (3-OH OME). Route of Elimination: Urinary excretion is a primary route of excretion of omeprazole metabolites. Little, if any unchanged drug was excreted in the urine. The majority of the dose (about 77%) was eliminated in urine as at least six metabolites. Two were identified as hydroxyomeprazole and the corresponding carboxylic acid. The remainder of the dose was recovered in the feces. Half Life: 0.5-1 hour (healthy subjects, delayed-release capsule); 3 hours (hepatic impairment) Biological Half-Life 0.5-1 hour (healthy subjects, delayed-release capsule) Approximately 3 hours (hepatic impairment) Plasma - Normal hepatic function - 30 minutes to 1 hour. Chronic hepatic disease - 3 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Despite their wide use, omeprazole and esomeprazole have only rarely been associated with hepatic injury. In large scale, long term trials , serum ALT elevations occurred in less than 1% of patients and at rates similar to those that occurred with placebo or comparator drugs. A small number of cases of clinically apparent liver disease due to omeprazole or esomeprazole have been published, the frequency of these cases probably being less than 1:100,000 users. A somewhat characteristic clinical phenotype has been described, with most cases arising during the first 1 to 4 weeks of therapy and being marked by an acute hepatocellular pattern of injury, with rapid recovery upon withdrawal. Rash, fever and eosinophilia were rare, as is autoantibody formation. Liver biopsy typically shows prominent centrolobular necrosis, suggestive of an acute, toxic hepatic injury (acute hepatic necrosis); however, recurrence upon rechallenge has been documented in several cases. In some instances, other organ involvement is prominent including rhabdomyolysis, lactic acidosis, renal insufficiency or Stevens Johnson syndrome. In large case series of drug induced liver injury, omeprazole and esomeprazole have accounted for few instances of symptomatic acute liver injury and rare instances of acute liver failure. Likelihood score: B (rare but likely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited information indicates that maternal omeprazole doses of 20 mg daily produce low levels in milk and would not be expected to cause any adverse effects in breastfed infants. ◉ Effects in Breastfed Infants One mother taking oral omeprazole 20 mg daily pumped and discarded her milk once each day 4 hours after her morning dose. She breastfed her infant the remainder of the day for 3 months before weaning. The infant remained well at 12 months of age. ◉ Effects on Lactation and Breastmilk The Spanish pharmacovigilance system found 20 cases of gynecomastia reported in patients taking omeprazole during the time period of 1982 to 2006. A retrospective claims database study in the United States found that users of proton pump inhibitors had an increased risk of gynecomastia. A review article reported that a search of database from the European Pharmacovigilance Centre found 104 cases of gynecomastia, 15 cases of galactorrhea, 15 cases of breast pain and 16 cases of breast enlargement associated with omeprazole. A search of the WHO global pharmacovigilance database found 439 cases of gynecomastia, 46 cases of galactorrhea, 93 cases of breast pain and 63 cases of breast enlargement associated with omeprazole. A 13-year-old girl was placed on omeprazole 20 mg twice daily by mouth for dyspepsia caused by mefenamic acid and a Helicobacter pylori infection. After 2 days of therapy, she developed bilateral galactorrhea and elevated serum prolactin. Three weeks after discontinuing omeprazole, galactorrhea and hyperprolactinemia resolved. Six weeks later, she was rechallenged with omeprazole and her serum prolactin rose from 27 to 70 mcg/L. Prolactin returned to normal 2 weeks after omeprazole discontinuation. Over the next 6 months, she was given domperidone on one occasion and lansoprazole on another. With both drugs, she developed galactorrhea and hyperprolactinemia which returned to normal after drug discontinuation. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. A 26-year-old woman with a kidney transplant developed galactorrhea after her kidney function decreased. She was taking tacrolimus, prednisone, amlodipine, labetalol, lovastatin, nortriptyline and pyridoxine as well as omeprazole for heartburn. Her omeprazole dose had been increased from 20 mg twice a day to 40 mg twice a day 3 months prior. A week earlier, she had been given prescriptions for naratriptan for migraine and metoclopramide for nausea at an emergency department visit. Her symptoms persisted 4 weeks later and her serum prolactin was elevated. Metoclopramide was discontinued with no improvement change in serum prolactin. Omeprazole was discontinued and calcium carbonate started. Two weeks later, her serum prolactin had normalized. Two months later, her heartburn increased and omeprazole was restarted at 20 mg daily with no increase in serum prolactin. The patient’s hyperprolactinemia and galactorrhea were probably caused by omeprazole. A 26-year-old Bhutanese woman with a kidney transplant was maintained on tacrolimus 2 mg twice daily, prednisolone 5 mg once daily, leflunomide 20 mg once daily, nifedipine 40 mg twice daily, and hydralazine 50 mg three times daily. She came to the emergency room with a complaint of abdominal pain. Her tacrolimus dose was adjusted, and oral omeprazole was begun. After 3 days, she experienced milk production from her left breast. According to the patient she had experienced the same reaction 7 years prior when she had her kidney transplant. Omeprazole was stopped and milk production ceased 3 days later. The authors rated the galactorrhea as definitely being caused by omeprazole. ◉ Summary of Use during Lactation Esomeprazole is the S-enantiomer of the proton-pump inhibitor, omeprazole. Limited information indicates that maternal doses of 10 mg daily produce low levels in milk and would not be expected to cause any adverse effects in breastfed infants. ◉ Effects in Breastfed Infants One mother taking omeprazole 20 mg daily orally pumped and discarded her milk once each day 4 hours after her morning dose. She breastfed her infant the remainder of the day for 3 months before weaning. The infant remained well at 12 months of age. A woman with rheumatoid arthritis was treated with oral esomeprazole 10 mg, prednisone 2.5 mg and sulfasalazine 1 gram once daily as well as injections of certolizumab pegol 200 mg every 2 weeks. Her infant was about 50% breastfed and 50% formula fed. The infant had no detectable drug-related adverse effects. ◉ Effects on Lactation and Breastmilk Omeprazole (the racemic form) has been reported to cause gynecomastia in several men and a retrospective claims database study in the United States found that users of proton pump inhibitors had an increased risk of gynecomastia. A review article reported that a search of database from the European Pharmacovigilance Centre found 45 cases of gynecomastia, 9 cases of galactorrhea, 19 cases of breast pain and 12 cases of breast enlargement associated with esomeprazole. A search of the WHO global pharmacovigilance database found 114 cases of gynecomastia, 38 cases of galactorrhea, 56 cases of breast pain and 28 cases of breast enlargement associated with esomeprazole. One woman developed elevated serum prolactin and estradiol with bilateral galactorrhea one week after starting esomeprazole 40 mg once daily for reflux esophagitis. The galactorrhea disappeared 3 days after discontinuing esomeprazole and prolactin and estradiol returned to normal 7 days after discontinuation. One month later, the patient restarted esomeprazole and again developed bilateral galactorrhea. She was switched to lansoprazole with no galactorrhea developing. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding Approximately 95% bound to human plasma proteins. |
| 参考文献 | |
| 其他信息 |
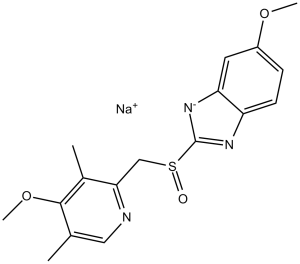
6-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methylsulfinyl]-1H-benzimidazole is a member of benzimidazoles and a sulfoxide.
Originally approved by the FDA in 1989, omeprazole is a proton-pump inhibitor, used to treat gastric acid-related disorders. These disorders may include gastroesophageal reflux disease (GERD), peptic ulcer disease, and other diseases characterized by the oversecretion of gastric acid. This drug was the first clinical useful drug in its class, and its approval was followed by the formulation of many other proton pump inhibitor drugs. Omeprazole is generally effective and well-tolerated, promoting its popular use in children and adults. Omeprazole is a Proton Pump Inhibitor. The mechanism of action of omeprazole is as a Proton Pump Inhibitor, and Cytochrome P450 2C19 Inhibitor. Omeprazole and esomeprazole are proton pump inhibitors (PPIs) and potent inhibitor of gastric acidity which are widely used in the therapy of gastroesophageal reflux and peptic ulcer disease. Omeprazole and esomeprazole therapy are both associated with a low rate of transient and asymptomatic serum aminotransferase elevations and are rare causes of clinically apparent liver injury. Omeprazole is a benzimidazole with selective and irreversible proton pump inhibition activity. Omeprazole forms a stable disulfide bond with the sulfhydryl group of the hydrogen-potassium (H+ - K+) ATPase found on the secretory surface of parietal cells, thereby inhibiting the final transport of hydrogen ions (via exchange with potassium ions) into the gastric lumen and suppressing gastric acid secretion. This agent exhibits no anticholinergic activities and does not antagonize histamine H2 receptors. Omeprazole is a highly effective inhibitor of gastric acid secretion used in the therapy of stomach ulcers, dyspepsia, peptic ulcer disease , gastroesophageal reflux disease and Zollinger-Ellison syndrome. The drug inhibits the H(+)-K(+)-ATPase (H(+)-K(+)-exchanging ATPase) in the proton pump of Gastric Parietal Cells.--Pubchem. Omeprazole is one of the most widely prescribed drugs internationally and is available over the counter in some countries. A 4-methoxy-3,5-dimethylpyridyl, 5-methoxybenzimidazole derivative of timoprazole that is used in the therapy of STOMACH ULCERS and ZOLLINGER-ELLISON SYNDROME. The drug inhibits an H(+)-K(+)-EXCHANGING ATPASE which is found in GASTRIC PARIETAL CELLS. See also: Omeprazole Magnesium (has salt form); Omeprazole Sodium (has salt form); Omeprazole; Sodium Bicarbonate (component of) ... View More ... Drug Indication Omeprazole, according to the FDA label is a proton pump inhibitor (PPI) used for the following purposes: • Treatment of active duodenal ulcer in adults • Eradication of Helicobacter pylori to reduce the risk of duodenal ulcer recurrence in adults • Treatment of active benign gastric ulcer in adults • Reduction of risk of upper gastrointestinal (GI) bleeding in critically ill adult patients. • Treatment of symptomatic gastroesophageal reflux disease (GERD) in patients 1 year of age and older • Treatment of erosive esophagitis (EE) due to acid-mediated GERD in patients 1 month of age and older • Maintenance of healing of EE due to acid-mediated GERD in patients 1 year of age and older • Pathologic hypersecretory conditions in adults FDA Label Mechanism of Action Hydrochloric acid (HCl) secretion into the gastric lumen is a process regulated mainly by the H(+)/K(+)-ATPase of the proton pump, expressed in high quantities by the parietal cells of the stomach. ATPase is an enzyme on the parietal cell membrane that facilitates hydrogen and potassium exchange through the cell, which normally results in the extrusion of potassium and formation of HCl (gastric acid). Omeprazole is a member of a class of antisecretory compounds, the substituted _benzimidazoles_, that stop gastric acid secretion by selective inhibition of the _H+/K+ ATPase_ enzyme system. Proton-pump inhibitors such as omeprazole bind covalently to cysteine residues via disulfide bridges on the alpha subunit of the _H+/K+ ATPase_ pump, inhibiting gastric acid secretion for up to 36 hours. This antisecretory effect is dose-related and leads to the inhibition of both basal and stimulated acid secretion, regardless of the stimulus. **Mechanism of H. pylori eradication** Peptic ulcer disease (PUD) is frequently associated with _Helicobacter pylori_ bacterial infection (NSAIDs). The treatment of H. pylori infection may include the addition of omeprazole or other proton pump inhibitors as part of the treatment regimen,. _H. pylori_ replicates most effectively at a neutral pH. Acid inhibition in H. pylori eradication therapy, including proton-pump inhibitors such as omeprazole, raises gastric pH, discouraging the growth of H.pylori. It is generally believed that proton pump inhibitors inhibit the _urease_ enzyme, which increases the pathogenesis of H. pylori in gastric-acid related conditions. Omeprazole is a selective and irreversible proton pump inhibitor. Omeprazole suppresses gastric acid secretion by specific inhibition of the hydrogen-potassium adenosinetriphosphatase (H+, K+-ATPase) enzyme system found at the secretory surface of parietal cells. It inhibits the final transport of hydrogen ions (via exchange with potassium ions) into the gastric lumen. Since the H+/K+ ATPase enzyme system is regarded as the acid (proton) pump of the gastric mucosa, omeprazole is known as a gastric acid pump inhibitor. Omeprazole inhibits both basal and stimulated acid secretion irrespective of the stimulus. After oral administration, the onset of the antisecretory effect of omeprazole occurs within one hour, with the maximum effect occurring within two hours. Inhibition of secretion is about 50% of maximum at 24 hours and the duration of inhibition lasts up to 72 hours. The antisecretory effect thus lasts far longer than would be expected from the very short (less than one hour) plasma half-life, apparently due to prolonged binding to the parietal H + /K + ATPase enzyme. When the drug is discontinued, secretory activity returns gradually, over 3 to 5 days. The inhibitory effect of omeprazole on acid secretion increases with repeated once-daily dosing, reaching a plateau after four days. Esomeprazole is a proton pump inhibitor that suppresses gastric acid secretion by specific inhibition of the H+/K+-ATPase in the gastric parietal cell. The S- and R-isomers of omeprazole are protonated and converted in the acidic compartment of the parietal cell forming the active inhibitor, the achiral sulphenamide. By acting specifically on the proton pump, esomeprazole blocks the final step in acid production, thus reducing gastric acidity. This effect is dose-related up to a daily dose of 20 to 40 mg and leads to inhibition of gastric acid secretion. |
| 分子式 |
C17H18N3O3S.NA
|
|
|---|---|---|
| 分子量 |
367.4
|
|
| 精确质量 |
367.096
|
|
| CAS号 |
161796-78-7
|
|
| 相关CAS号 |
Esomeprazole;119141-88-7;Esomeprazole magnesium trihydrate;217087-09-7;Esomeprazole magnesium;161973-10-0;Esomeprazole magnesium salt;1198768-91-0;Esomeprazole potassium salt;161796-84-5;Esomeprazole hemistrontium;914613-86-8;Esomeprazole-d6 sodium;922731-04-2
|
|
| PubChem CID |
4594
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 沸点 |
600ºC at 760 mmHg
|
|
| 闪点 |
316.7ºC
|
|
| LogP |
3.551
|
|
| tPSA |
85.45
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
24
|
|
| 分子复杂度/Complexity |
453
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
RYXPMWYHEBGTRV-JIDHJSLPSA-N
|
|
| InChi Code |
InChI=1S/C17H18N3O3S.Na/c1-10-8-18-15(11(2)16(10)23-4)9-24(21)17-19-13-6-5-12(22-3)7-14(13)20-17;/h5-8H,9H2,1-4H3;/q-1;+1/t24-;/m0./s1
|
|
| 化学名 |
sodium (S)-5-methoxy-2-(((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)sulfinyl)benzo[d]imidazol-1-ide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: (1). 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 (2). 该产品在溶液状态不稳定,请现配现用。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.66 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.66 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (5.66 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7218 mL | 13.6091 mL | 27.2183 mL | |
| 5 mM | 0.5444 mL | 2.7218 mL | 5.4437 mL | |
| 10 mM | 0.2722 mL | 1.3609 mL | 2.7218 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。