| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 10g |
|
||
| Other Sizes |
|

| 体外研究 (In Vitro) |
埃索美拉唑镁是一种 H+、K+-ATP 酶抑制剂[1]。
|
|---|---|
| 体内研究 (In Vivo) |
埃索美拉唑镁治疗(0.5–50 mg/kg;口服灌胃;每天;持续 10 天;A/J 小鼠)可提高铜/锌超氧化物歧化酶的活性和胃的总抗氧化能力[1]。
|
| 动物实验 |
Animal/Disease Models: A/J mice[1]
Doses: 0.5 mg/kg, 5 mg/kg, 50 mg/kg Route of Administration: po (oral gavage); daily; for 10 days Experimental Results: Gastric total antioxidant capacity and Cu/Zn-superoxide dismutase activity are increased. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
After oral administration, peak plasma levels (Cmax) occur at approximately 1.5 hours (Tmax). The Cmax increases proportionally when the dose is increased, and there is a three-fold increase in the area under the plasma concentration-time curve (AUC) from 20 to 40 mg. At repeated once-daily dosing with 40 mg, the systemic bioavailability is approximately 90% compared to 64% after a single dose of 40 mg. The mean exposure (AUC) to esomeprazole increases from 4.32 μmol*hr/L on Day 1 to 11.2 μmol*hr/L on Day 5 after 40 mg once daily dosing. The AUC after administration of a single 40 mg dose of Esomeprazole is decreased by 43% to 53% after food intake compared to fasting conditions. Esomeprazole should be taken at least one hour before meals. _Combination Therapy with Antimicrobials:_ Esomeprazole magnesium 40 mg once daily was given in combination with [DB01211] 500 mg twice daily and [DB01060] 1000 mg twice daily for 7 days to 17 healthy male and female subjects. The mean steady state AUC and Cmax of esomeprazole increased by 70% and 18%, respectively during triple combination therapy compared to treatment with esomeprazole alone. The observed increase in esomeprazole exposure during co-administration with clarithromycin and amoxicillin is not expected to produce significant safety concerns. The plasma elimination half-life of esomeprazole is approximately 1 to 1.5 hours. Less than 1% of parent drug is excreted in the urine. Approximately 80% of an oral dose of esomeprazole is excreted as inactive metabolites in the urine, and the remainder is found as inactive metabolites in the feces. The apparent volume of distribution at steady state in healthy volunteers is approximately 16 L. The plasma elimination half-life of esomeprazole is approximately 1 to 1.5 hours. Less than 1% of parent drug is excreted in the urine. Approximately 80% of an oral dose of esomeprazole is excreted as inactive metabolites in the urine, and the remainder is found as inactive metabolites in the feces. Esomeprazole is 97% bound to plasma proteins. Plasma protein binding is constant over the concentration range of 2 to 20 umol/L. The apparent volume of distribution at steady state in healthy volunteers is approximately 16 L. NEXIUM Delayed-Release Capsules and NEXIUM For Delayed-Release Oral Suspension contain a bioequivalent enteric-coated granule formulation of esomeprazole magnesium. Bioequivalency is based on a single dose (40 mg) study in 94 healthy male and female volunteers under fasting condition. After oral administration peak plasma levels (Cmax) occur at approximately 1.5 hours (Tmax). The Cmax increases proportionally when the dose is increased, and there is a three-fold increase in the area under the plasma concentration-time curve (AUC) from 20 to 40 mg. At repeated once-daily dosing with 40 mg, the systemic bioavailability is approximately 90% compared to 64% after a single dose of 40 mg. The mean exposure (AUC) to esomeprazole increases from 4.32 umol*hr/L on Day 1 to 11.2 umol*hr/L on Day 5 after 40 mg once daily dosing. Metabolism / Metabolites Esomeprazole is extensively metabolized in the liver by the cytochrome P450 (CYP) enzyme system. The metabolites of esomeprazole lack antisecretory activity. The major part of esomeprazole’s metabolism is dependent upon the CYP2C19 isoenzyme, which forms the hydroxy and desmethyl metabolites. The remaining amount is dependent on CYP3A4 which forms the sulphone metabolite. CYP2C19 isoenzyme exhibits polymorphism in the metabolism of esomeprazole, since some 3% of Caucasians and 15 to 20% of Asians lack CYP2C19 and are termed Poor Metabolizers. However, the influence of CYP 2C19 polymorphism is less pronounced for esomeprazole than for omeprazole. At steady state, the ratio of AUC in Poor Metabolizers to AUC in the rest of the population (Extensive Metabolizers) is approximately 2. Following administration of equimolar doses, the S- and R-isomers are metabolized differently by the liver, resulting in higher plasma levels of the S- than of the R-isomer. Nine major urinary metabolites have been detected. The two main metabolites have been identified as hydroxyesomeprazole and the corresponding carboxylic acid. Three major metabolites have been identified in plasma: the 5-O-desmethyl- and sulphone derivatives and hydroxyesomeprazole. The major metabolites of esomeprazole have no effect on gastric acid secretion. Esomeprazole is extensively metabolized in the liver by the cytochrome P450 (CYP) enzyme system. The metabolites of esomeprazole lack antisecretory activity. The major part of esomeprazole's metabolism is dependent upon the CYP 2C19 isoenzyme, which forms the hydroxy and desmethyl metabolites. The remaining amount is dependent on CYP 3A4 which forms the sulphone metabolite. CYP 2C19 isoenzyme exhibits polymorphism in the metabolism of esomeprazole, since some 3% of Caucasians and 15 to 20% of Asians lack CYP 2C19 and are termed Poor Metabolizers. At steady state, the ratio of AUC in Poor Metabolizers to AUC in the rest of the population (Extensive Metabolizers) is approximately 2. Following administration of equimolar doses, the S- and R-isomers are metabolized differently by the liver, resulting in higher plasma levels of the S- than of the R-isomer. Biological Half-Life 1-1.5 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Esomeprazole is the S-enantiomer of the proton-pump inhibitor, omeprazole. Limited information indicates that maternal doses of 10 mg daily produce low levels in milk and would not be expected to cause any adverse effects in breastfed infants. ◉ Effects in Breastfed Infants One mother taking omeprazole 20 mg daily orally pumped and discarded her milk once each day 4 hours after her morning dose. She breastfed her infant the remainder of the day for 3 months before weaning. The infant remained well at 12 months of age. A woman with rheumatoid arthritis was treated with oral esomeprazole 10 mg, prednisone 2.5 mg and sulfasalazine 1 gram once daily as well as injections of certolizumab pegol 200 mg every 2 weeks. Her infant was about 50% breastfed and 50% formula fed. The infant had no detectable drug-related adverse effects. ◉ Effects on Lactation and Breastmilk Omeprazole (the racemic form) has been reported to cause gynecomastia in several men and a retrospective claims database study in the United States found that users of proton pump inhibitors had an increased risk of gynecomastia. A review article reported that a search of database from the European Pharmacovigilance Centre found 45 cases of gynecomastia, 9 cases of galactorrhea, 19 cases of breast pain and 12 cases of breast enlargement associated with esomeprazole. A search of the WHO global pharmacovigilance database found 114 cases of gynecomastia, 38 cases of galactorrhea, 56 cases of breast pain and 28 cases of breast enlargement associated with esomeprazole. One woman developed elevated serum prolactin and estradiol with bilateral galactorrhea one week after starting esomeprazole 40 mg once daily for reflux esophagitis. The galactorrhea disappeared 3 days after discontinuing esomeprazole and prolactin and estradiol returned to normal 7 days after discontinuation. One month later, the patient restarted esomeprazole and again developed bilateral galactorrhea. She was switched to lansoprazole with no galactorrhea developing. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding Esomeprazole is 97% bound to plasma proteins. Plasma protein binding is constant over the concentration range of 2 to 20 µmol/L. |
| 参考文献 | |
| 其他信息 |
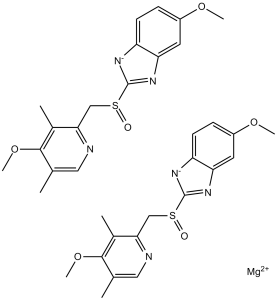
Esomeprazole magnesium is a magnesium salt resulting from the formal reaction of magnesium hydroxide with 2 mol eq. of esomeprazole. An inhibitor of gastric acid secretion, it is used for the treatment of gastro-oesophageal reflux disease, dyspepsia, peptic ulcer disease, and Zollinger-Ellison syndrome. It has a role as an EC 3.6.3.10 (H(+)/K(+)-exchanging ATPase) inhibitor and an anti-ulcer drug. It contains an esomeprazole(1-).
Esomeprazole, sold under the brand name Nexium, is a proton pump inhibitor (PPI) medication used for the management of gastroesophageal reflux disease (GERD), for gastric protection to prevent recurrence of stomach ulcers or gastric damage from chronic use of NSAIDs, and for the treatment of pathological hypersecretory conditions including Zollinger-Ellison (ZE) Syndrome. It can also be found in quadruple regimens for the treatment of H. pylori infections along with other antibiotics including [DB01060], [DB01211], and [DB00916], for example. Its efficacy is considered similar to other medications within the PPI class including [DB00338], [DB00213], [DB00448], [DB05351], and [DB01129]. Esomeprazole is the s-isomer of [DB00338], which is a racemate of the S- and R-enantiomer. Esomeprazole has been shown to inhibit acid secretion to a similar extent as [DB00338], without any significant differences between the two compounds in vitro. Esomeprazole exerts its stomach acid-suppressing effects by preventing the final step in gastric acid production by covalently binding to sulfhydryl groups of cysteines found on the (H+, K+)-ATPase enzyme at the secretory surface of gastric parietal cells. This effect leads to inhibition of both basal and stimulated gastric acid secretion, irrespective of the stimulus. As the binding of esomeprazole to the (H+, K+)-ATPase enzyme is irreversible and new enzyme needs to be expressed in order to resume acid secretion, esomeprazole's duration of antisecretory effect persists longer than 24 hours. PPIs such as esomeprazole have also been shown to inhibit the activity of dimethylarginine dimethylaminohydrolase (DDAH), an enzyme necessary for cardiovascular health. DDAH inhibition causes a consequent accumulation of the nitric oxide synthase inhibitor asymmetric dimethylarginie (ADMA), which is thought to cause the association of PPIs with increased risk of cardiovascular events in patients with unstable coronary syndromes. Due to their good safety profile and as several PPIs are available over the counter without a prescription, their current use in North America is widespread. Long term use of PPIs such as esomeprazole has been associated with possible adverse effects, however, including increased susceptibility to bacterial infections (including gastrointestinal C. difficile), reduced absorption of micronutrients such as iron and B12, and an increased risk of developing hypomagnesemia and hypocalcemia which may contribute to osteoporosis and bone fractures later in life. Rapid discontinuation of PPIs such as esomeprazole may cause a rebound effect and a short term increase in hypersecretion. Esomeprazole doses should be slowly lowered, or tapered, before discontinuing to prevent this rebound effect. Esomeprazole Magnesium is the magnesium salt of esomeprazole, the S-isomer of omeprazole, with gastric proton pump inhibitor activity. In the acidic compartment of parietal cells, esomeprazole is protonated and converted into the active achiral sulphenamide; the active sulphenamide forms one or more covalent disulfide bonds with the proton pump hydrogen-potassium adenosine triphosphatase (H+/K+ ATPase), thereby inhibiting its activity and the parietal cell secretion of H+ ions into the gastric lumen, the final step in gastric acid production. H+/K+ ATPase is an integral membrane protein of the gastric parietal cell. Esomeprazole is the S-isomer of omeprazole, with gastric proton pump inhibitor activity. In the acidic compartment of parietal cells, esomeprazole is protonated and converted into the active achiral sulfenamide; the active sulfenamide forms one or more covalent disulfide bonds with the proton pump hydrogen-potassium adenosine triphosphatase (H+/K+ ATPase), thereby inhibiting its activity and the parietal cell secretion of H+ ions into the gastric lumen, the final step in gastric acid production. H+/K+ ATPase is an integral membrane protein of the gastric parietal cell. The S-isomer of omeprazole. See also: Esomeprazole (has active moiety); Esomeprazole Magnesium; Naproxen (component of). Drug Indication Esomeprazole is indicated for the treatment of acid-reflux disorders including healing and maintenance of erosive esophagitis, and symptomatic gastroesophageal reflux disease (GERD), peptic ulcer disease, H. pylori eradication, prevention of gastrointestinal bleeds with NSAID use, and for the long-term treatment of pathological hypersecretory conditions including Zollinger-Ellison Syndrome. FDA Label Nexium Control is indicated for the short-term treatment of reflux symptoms (e. g. heartburn and acid regurgitation) in adults. Mechanism of Action Esomeprazole exerts its stomach acid-suppressing effects by preventing the final step in gastric acid production by covalently binding to sulfhydryl groups of cysteines found on the (H+, K+)-ATPase enzyme at the secretory surface of gastric parietal cells. This effect leads to inhibition of both basal and stimulated gastric acid secretion, irrespective of the stimulus. As the binding of esomeprazole to the (H+, K+)-ATPase enzyme is irreversible and new enzyme needs to be expressed in order to resume acid secretion, esomeprazole's duration of antisecretory effect that persists longer than 24 hours. Esomeprazole is a proton pump inhibitor that suppresses gastric acid secretion by specific inhibition of the H+/K+-ATPase in the gastric parietal cell. The S- and R-isomers of omeprazole are protonated and converted in the acidic compartment of the parietal cell forming the active inhibitor, the achiral sulphenamide. By acting specifically on the proton pump, esomeprazole blocks the final step in acid production, thus reducing gastric acidity. This effect is dose-related up to a daily dose of 20 to 40 mg and leads to inhibition of gastric acid secretion. |
| 分子式 |
C34H36MGN6O6S2
|
|
|---|---|---|
| 分子量 |
713.12
|
|
| 精确质量 |
712.198
|
|
| CAS号 |
161973-10-0
|
|
| 相关CAS号 |
Esomeprazole;119141-88-7;Esomeprazole magnesium trihydrate;217087-09-7;Esomeprazole sodium;161796-78-7;Omeprazole magnesium;95382-33-5;Esomeprazole magnesium salt;1198768-91-0;Esomeprazole potassium salt;161796-84-5;Esomeprazole hemistrontium;914613-86-8
|
|
| PubChem CID |
9568613
|
|
| 外观&性状 |
Light yellow to yellow solid powder
|
|
| 沸点 |
600ºC at 760 mmHg
|
|
| 闪点 |
316.7ºC
|
|
| 蒸汽压 |
2.35E-14mmHg at 25°C
|
|
| LogP |
6.789
|
|
| tPSA |
186.82
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
14
|
|
| 可旋转键数目(RBC) |
10
|
|
| 重原子数目 |
49
|
|
| 分子复杂度/Complexity |
453
|
|
| 定义原子立体中心数目 |
2
|
|
| InChi Key |
KWORUUGOSLYAGD-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/2C17H18N3O3S.Mg/c2*1-10-8-18-15(11(2)16(10)23-4)9-24(21)17-19-13-6-5-12(22-3)7-14(13)20-17;/h2*5-8H,9H2,1-4H3;/q2*-1;+2
|
|
| 化学名 |
magnesium;5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl]benzimidazol-1-ide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (2.92 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (2.92 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (2.92 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 1.43 mg/mL (2.01 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4023 mL | 7.0114 mL | 14.0229 mL | |
| 5 mM | 0.2805 mL | 1.4023 mL | 2.8046 mL | |
| 10 mM | 0.1402 mL | 0.7011 mL | 1.4023 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。