| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
通过增加细胞内酸度,埃索美拉唑(25-100 µM;20 小时;MDA-MB-468 细胞)疗法以剂量依赖性方式抑制三阴性乳腺癌细胞的体外增殖 [1]。
|
|---|---|
| 体内研究 (In Vivo) |
用埃索美拉唑(30-300 mg/kg;口服灌胃;每天;持续 19 或 11 天)治疗的 C57BL/6J 小鼠显示出动物肺纤维化进展显着减少。此外,埃索美拉唑还可降低循环纤维化和炎症标志物[2]。
|
| 细胞实验 |
细胞活力测定[1]
细胞类型: MDA-MB-468 细胞 测试浓度: 25 µM、50 µM、75 µM、100 µM 孵育持续时间:20小时 实验结果:在体外以剂量依赖性方式抑制三阴性乳腺癌细胞。 |
| 动物实验 |
Animal/Disease Models: C57BL/6J mice (8 weeks old, 25-30 g) cotton smoke-induced lung injury [2]
Doses: 30 mg/kg, 300 mg/kg Route of Administration: po (oral gavage); daily; continued for 19 Or 11-day Experimental Results: Dramatically inhibited the progression of lung fibrosis in animals. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Omeprazole delayed-release capsules contain an enteric-coated granule formulation of omeprazole (because omeprazole is acid-labile), so that absorption of omeprazole begins only after the granules exit the stomach. Absorption of omeprazole occurs rapidly, with peak plasma concentrations of omeprazole achieved within 0.5-3.5 hours. Absolute bioavailability (compared with intravenous administration) is approximately 30-40% at doses of 20-40 mg, largely due to pre-systemic metabolism. The bioavailability of omeprazole increases slightly upon repeated administration of omeprazole delayed-release capsules. After a single dose oral dose of a buffered solution of omeprazole, negligible (if any) amounts of unchanged drug were excreted in urine. Most of the dose (about 77%) was eliminated in urine as at least six different metabolites. Two metabolites were identified as _hydroxyomeprazole_ and the corresponding _carboxylic acid_. The remainder of the dose was found in the feces. This suggests significant biliary excretion of omeprazole metabolites. Three metabolites have been identified in the plasma, the _sulfide_ and _sulfone_ derivatives of omeprazole, and _hydroxyomeprazole_. These metabolites possess minimal or no antisecretory activity. Approximately 0.3 L/kg, corresponding to the volume of extracellular water. Healthy subject (delayed release capsule), total body clearance 500 - 600 mL/min Geriatric plasma clearance: 250 mL/min Hepatic impairment plasma clearance: 70 mL/min Absorption: rapid. Distribution: Distributed in tissue, particularly gastric parietal cells. Elimination: Renal 72 to 80%. Fecal 18 to 23%. In dialysis - Not readily dialyzable, because of extensive protein binding. To clarify the in vivo first-pass metabolism of omeprazole, the pharmacokinetics were examined after oral, intraduodenal, intraportal venous, and intravenous administration at various doses to rats. Extraction ratios in the liver and intestinal tract were determined from the areas under the concentration-time curve (AUC) for intraportal venous and intravenous administration and from those for intraduodenal and intraportal venous administration, respectively. Assuming that the drug was absorbed from the gastrointestinal tract completely, the hepatic and intestinal extraction ratios were 0.80, 0.63, and 0.59 at doses of 2.5, 5, and 10 mg/kg and 0.70 and 0.73 at doses of 5 and 10 mg/kg, respectively. The bioavailability of orally administered omeprazole was 6.4, 9.6, and 12.6% at the doses of l0, 20, and 40 mg/kg, respectively. There were no differences in the distribution volume of steady state, total clearance, or elimination half-life at any doses. In addition, the AUC value after oral administration (20 mg/kg) in rats acutely intoxicated with CC(14) was 2.4 times larger than that in the control. These findings suggest that omeprazole undergoes a first-pass metabolism in the intestinal mucosa and/or lumen, as well as in the liver, and that the major contribution to the dose-dependent increase in bioavailability is a saturation of the first-pass metabolism in the liver. Omeprazole is distributed into human milk; following oral administration of omeprazole 20 mg in one lactating women, concentrations of the drug were measured in breast milk. For more Absorption, Distribution and Excretion (Complete) data for OMEPRAZOLE (6 total), please visit the HSDB record page. The plasma elimination half-life of esomeprazole is approximately 1 to 1.5 hours. Less than 1% of parent drug is excreted in the urine. Approximately 80% of an oral dose of esomeprazole is excreted as inactive metabolites in the urine, and the remainder is found as inactive metabolites in the feces. Esomeprazole is 97% bound to plasma proteins. Plasma protein binding is constant over the concentration range of 2 to 20 umol/L. The apparent volume of distribution at steady state in healthy volunteers is approximately 16 L. NEXIUM Delayed-Release Capsules and NEXIUM For Delayed-Release Oral Suspension contain a bioequivalent enteric-coated granule formulation of esomeprazole magnesium. Bioequivalency is based on a single dose (40 mg) study in 94 healthy male and female volunteers under fasting condition. After oral administration peak plasma levels (Cmax) occur at approximately 1.5 hours (Tmax). The Cmax increases proportionally when the dose is increased, and there is a three-fold increase in the area under the plasma concentration-time curve (AUC) from 20 to 40 mg. At repeated once-daily dosing with 40 mg, the systemic bioavailability is approximately 90% compared to 64% after a single dose of 40 mg. The mean exposure (AUC) to esomeprazole increases from 4.32 umol*hr/L on Day 1 to 11.2 umol*hr/L on Day 5 after 40 mg once daily dosing. Metabolism / Metabolites Omeprazole is heavily metabolized in the liver by the cytochrome P450 (CYP) enzyme system. The main part of its metabolism depends on the polymorphically expressed CYP2C19, which is responsible for the formation of _hydroxyomeprazole_, the major metabolite found in plasma. The remaining part depends on CYP3A4, responsible for the formation of _omeprazole sulphone_. The in vitro metabolism of omeprazole was studied in human liver microsomes in order to define the metabolic pathways and identify the cytochrome P450 (CYP) isoforms responsible for the formation of the major omeprazole metabolites. 2 The four major metabolites identified in vitro, in tentative order of importance, were hydroxyomeprazole, omeprazole sulphone, 5-O-desmethylomeprazole, and an unidentified compound termed metabolite X. Omeprazole pyridone was also detected but could not be quantitated. Incubation of hydroxyomeprazole and omeprazole sulphone with human microsomes resulted in both cases in formation of the hydroxysulphone. The kinetics of formation of the four primary metabolites studied were biphasic suggesting the involvement of multiple CYP isoforms in each case. Further studies used substrate concentrations at which the high affinity activities predominated. 3 Formation of the major metabolite, hydroxyomeprazole, was significantly correlated with S-mephenytoin hydroxylase and with benzo[a]pyrene metabolism and CYP3A content. Inhibition studies with isoform selective inhibitors also indicated a dominant role of S-mephenytoin hydroxylase with some CYP3A contribution in the formation of hydroxyomeprazole. Correlation and inhibition data for the sulphone and metabolite X were consistent with a predominant role of the CYP3A subfamily in formation of these metabolites. Formation of 5-O-desmethylomeprazole was inhibited by both R, S-mephenytoin and quinidine, indicating that both S-mephenytoin hydroxylase and CYP2D6 may mediate this reaction in human liver microsomes and in vivo. The Vmax/Km (indicator of intrinsic clearance in vivo) for hydroxyomeprazole was four times greater than that for omeprazole sulphone. Consistent with findings in vivo, the results predict that omeprazole clearance in vivo would be reduced in poor metabolisers of mephenytoin due to reduction in the dominant partial metabolic clearance to hydroxyomeprazole. Esomeprazole is extensively metabolized in the liver by the cytochrome P450 (CYP) enzyme system. The metabolites of esomeprazole lack antisecretory activity. The major part of esomeprazole's metabolism is dependent upon the CYP 2C19 isoenzyme, which forms the hydroxy and desmethyl metabolites. The remaining amount is dependent on CYP 3A4 which forms the sulphone metabolite. CYP 2C19 isoenzyme exhibits polymorphism in the metabolism of esomeprazole, since some 3% of Caucasians and 15 to 20% of Asians lack CYP 2C19 and are termed Poor Metabolizers. At steady state, the ratio of AUC in Poor Metabolizers to AUC in the rest of the population (Extensive Metabolizers) is approximately 2. Following administration of equimolar doses, the S- and R-isomers are metabolized differently by the liver, resulting in higher plasma levels of the S- than of the R-isomer. Omeprazole has known human metabolites that include 3-Hydroxyomeprazole, 5-Hydroxyomeprazole, Omeprazole sulfone, and 5'-O-Desmethyl omeprazole. Hepatic. Omeprazole is extensively metabolized by the cytochrome P450 (CYP) enzyme system. The two primary CYP isozymes involved are CYP2C19 and CYP3A4. Metabolism is stereoselective in which the S-isomer is converted to 5'O-desmethylomeprazole via CYP2C19. CYP3A4 converts the S-isomer to 3-hydroxyomeprazole. The R-isomer is converted to 5-hydroxyomeprazole by CYP2C19. CYP3A4 converts the R-isomer to any four different metabolites: 5-hydroxyomeprazole (5-OH OME), omeprazole sulfone (OME sulfone), 5'-O-desmethylomeprazole (5'-desmethyl OME), and 3-hydroxyomeprazole (3-OH OME). Route of Elimination: Urinary excretion is a primary route of excretion of omeprazole metabolites. Little, if any unchanged drug was excreted in the urine. The majority of the dose (about 77%) was eliminated in urine as at least six metabolites. Two were identified as hydroxyomeprazole and the corresponding carboxylic acid. The remainder of the dose was recovered in the feces. Half Life: 0.5-1 hour (healthy subjects, delayed-release capsule); 3 hours (hepatic impairment) Biological Half-Life 0.5-1 hour (healthy subjects, delayed-release capsule) Approximately 3 hours (hepatic impairment) Plasma - Normal hepatic function - 30 minutes to 1 hour. Chronic hepatic disease - 3 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Omeprazole is a proton pump inhibitor that suppresses gastric acid secretion by specific inhibition of the H+/K+-ATPase in the gastric parietal cell. By acting specifically on the proton pump, omeprazole blocks the final step in acid production, thus reducing gastric acidity. Interactions Inhibition of the cytochrome p450 enzyme system by omeprazole, especially in high dose, may cause a decrease in the hepatic metabolism of /coumarin- or indandione-derivative anticoagulants, diazepam or phenytoin/ which may result in delayed elimination and increased blood concentrations, when these medications are used concurrently with omeprazole. Omeprazole may increase gastrointestinal pH; concurrent use /of ampicillin esters, iron salts, or ketoconazole/ with omeprazole may result in a reduction in absorption of ampicillin esters, iron salts, or ketoconazole. Concurrent use of omeprazole with /bone marrow depressants/ may increase the leukopenic and/or thrombocytopenic effects of both these medications; if concurrent use is required, close observation for toxic effects should be considered. The effect of omeprazole on drug metabolism was studied using the model drugs antipyrine and (14)C-aminopyrine. Elimination of both model drugs was assessed before and after 15 days of therapy in male subjects. It was concluded that metabolic inhibition of the model drugs would not occur with normal clinical doses of omeprazole. For more Interactions (Complete) data for OMEPRAZOLE (9 total), please visit the HSDB record page. In a single-dose study, concomitant administration of omeprazole 20 mg and sucralfate 1 g resulted in delayed absorption of omeprazole and decreased omeprazole bioavailability by 16%. Proton-pump inhibitors should be administered at least 30 minutes before sucralfate. Pharmacokinetic interaction with omeprazole (decreased plasma concentrations and AUC of rilpivirine).32 343 Concomitant use of other proton-pump inhibitors also may result in decreased plasma concentration of rilpivirine.343 Concomitant use of rilpivirine and proton-pump inhibitors is contraindicated. Concomitant use of omeprazole 40 mg once daily and atazanavir (with or without low-dose ritonavir) results in a substantial decrease in plasma concentrations of atazanavir and possible loss of the therapeutic effect of the antiretroviral agent or development of drug resistance. Concomitant use of omeprazole 40 mg once daily (administered 2 hours before atazanavir) and atazanavir 400 mg once daily decreased the AUC and peak plasma concentration of atazanavir by 94 and 96%, respectively. The manufacturer of esomeprazole states that concomitant administration with atazanavir is not recommended. If atazanavir is administered in an antiretroviral treatment-naive patient receiving a proton-pump inhibitor, a ritonavir-boosted regimen of 300 mg of atazanavir once daily with ritonavir 100 mg once daily with food is recommended. The dose of the proton-pump inhibitor should be administered approximately 12 hours before ritonavir-boosted atazanavir; the dose of the proton-pump inhibitor should not exceed omeprazole 20 mg daily (or equivalent). Concomitant use of proton-pump inhibitors with atazanavir is not recommended in antiretroviral treatment-experienced patients. Concomitant use of omeprazole 20 mg once daily and digoxin in healthy individuals increased digoxin bioavailability by 10% (up to 30% in some individuals). Because esomeprazole is an enantiomer of omeprazole, concomitant use of esomeprazole with digoxin is expected to increase systemic exposure to digoxin; therefore, monitoring for manifestations of digoxin toxicity may be required during such concomitant use. For more Interactions (Complete) data for Esomeprazole (7 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse iv 0.08 g/kg LD50 Mouse oral >4 g/kg LD50 Rat iv >0.05 g/kg LD50 Rat oral >4 g/kg |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Anti-Ulcer Agents; Enzyme Inhibitors Omeprazole is indicated for the treatment of a complex of symptoms which may be caused by any of the conditions where a reduction of gastric acid secretion is required (e.g., duodenal ulcer, gastric ulcer, nonsteroidal anti-inflammatory drugs associated gastric and duodenal ulcer, reflux esophagitis, gastroesophageal reflex disease) or when no identifiable organic cause is found (i.e., functional dyspepsia). /Included in US product labeling/ Omeprazole is indicated for the treatment of heartburn and other symptoms associated with gastroesophageal reflux disease. Omeprazole is indicated for the short-term treatment of erosive esophagitis (associated with gastroesophageal reflux disease) that has been diagnosed by endoscopy. Omeprazole also is indicated to maintain healing of erosive esophagitis. /Included in US product labeling/ Omeprazole is indicated for the long-term treatment of pathologic gastric hypersecretion associated with Zollinger-Ellison syndrome (alone or as part of multiple endocrine neoplasia Type-1), systemic mastocytosis, and multiple endocrine adenoma. /Included in US product labeling/ For more Therapeutic Uses (Complete) data for OMEPRAZOLE (8 total), please visit the HSDB record page. Anti-Ulcer Agents; Proton Pump Inhibitors Although evidence currently is limited, proton-pump inhibitors have been used for gastric acid-suppressive therapy as an adjunct in the symptomatic treatment of upper GI Crohn's disease, including esophageal, gastroduodenal, and jejunoileal disease.22 23 24 25 26 27 28 Most evidence of efficacy to date has been from case studies in patients with Crohn's-associated peptic ulcer disease unresponsive to other therapies (e.g., H2-receptor antagonists, cytoprotective agents, antacids, and/or sucralfate). /Not included in product label/ Esomeprazole magnesium is used for the long-term treatment of pathologic GI hypersecretory conditions. Efficacy for this indication was established in an open-label study in a limited number of patients with previously diagnosed pathologic GI hypersecretory conditions (e.g., Zollinger-Ellison syndrome, idiopathic gastric acid hypersecretion); patients received total daily dosages of esomeprazole ranging from 80 mg-240 mg. The drug generally was well tolerated at these dosages for the duration of the study (12 months). At 12 months of therapy, 90% of patients treated with esomeprazole had controlled basal acid output (BAO) levels, defined as BAO of less than 5 or 10 mEq/hour in patients who had or had not previously undergone gastric acid-reducing surgery, respectively. Esomeprazole magnesium is used for reducing the occurrence of gastric ulcers associated with chronic nonsteroidal anti-inflammatory agent (NSAIA) therapy in patients at risk for developing these ulcers, including individuals 60 years of age or older and/or those with a documented history of gastric ulcers. Efficacy for this indication was established in two 6-month randomized, controlled studies in patients receiving chronic therapy with either a prototypical NSAIA or a selective cyclooxygenase-2 (COX-2) inhibitor; individuals enrolled in these studies were considered to be at risk for developing NSAIA-associated ulcers because of their age (60 years or older) and/or a history of documented gastric or duodenal ulcer within the previous 5 years, but they had no evidence of gastric or duodenal ulcers on endoscopic examination at the start of the studies. Results of the studies indicated that esomeprazole 20 or 40 mg daily was more effective than placebo in preventing gastric ulcer occurrence during 6 months of treatment; however, no additional benefit was observed with the 40-mg daily dosage compared with the 20-mg daily dosage. In these studies, 94.7-95.4% of patients receiving esomeprazole 20 mg daily, 95.3-96.7% of those receiving esomeprazole 40 mg daily, and 83.3-88.2% of those receiving placebo remained free of gastric ulcers, as determined by serial endoscopic examinations, throughout the 6-month study.1 The occurrence rate of duodenal ulcers was too low to determine the effect of esomeprazole therapy on duodenal ulcer occurrence. For more Therapeutic Uses (Complete) data for Esomeprazole (6 total), please visit the HSDB record page. Drug Warnings Pregnancy risk category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./ No information is available on the relationship of age to the effects of omeprazole in geriatric patients. However, a somewhat decreased rate of elimination and increased bioavailability are more likely to occur in geriatric patients taking omeprazole. Omeprazole generally is well tolerated. The most frequent adverse effects associated with omeprazole therapy involve the GI tract (e.g., diarrhea, nausea, constipation, abdominal pain, vomiting) and the CNS (e.g., headache, dizziness). Diarrhea, abdominal pain, nausea, vomiting, constipation, flatulence, and acid regurgitation are the most frequent adverse GI effects of omeprazole, occurring in about 1-5% of patients. Dysphagia, abdominal swelling, anorexia, irritable colon, fecal discoloration, pancreatitis (sometimes fatal), esophageal candidiasis, mucosal atrophy of the tongue, taste perversion, and dry mouth have been reported occasionally but in many cases were not directly attributed to the drug. Benign gastric fundic polyps have been reported rarely and appear to resolve upon discontinuation of omeprazole therapy. For more Drug Warnings (Complete) data for OMEPRAZOLE (15 total), please visit the HSDB record page. It is unknown whether esomeprazole is distributed into milk. However, omeprazole is distributed into human milk; discontinue nursing or drug because of potential risk in nursing infants. FDA Pregnancy Category: B /NO EVIDENCE OF RISK IN HUMANS. Adequate, well controlled studies in pregnant women have not shown increased risk of fetal abnormalities despite adverse findings in animals, or, in the absents of adequate human studies, animal studies show no fetal risk. The chance of fetal harm is remote but remains a possibility./ When esomeprazole is used in fixed combination with naproxen, the usual cautions, precautions, and contraindications associated with naproxen must be considered in addition to those associated with esomeprazole. Administration of proton-pump inhibitors has been associated with an increased risk for developing certain infections (e.g., community-acquired pneumonia). For more Drug Warnings (Complete) data for Esomeprazole (12 total), please visit the HSDB record page. Pharmacodynamics **Effects on gastric acid secretion** This drug decreases gastric acid secretion. After oral administration, the onset of the antisecretory effect of omeprazole is usually achieved within one hour, with the maximum effect occurring by 2 hours after administration. The inhibitory effect of omeprazole on acid secretion increases with repeated once-daily dosing, reaching a plateau after four days. **Effects on serum gastrin** In studies of 200 or more patients, serum gastrin levels increased during the first 1-2 weeks of daily administration of therapeutic doses of omeprazole. This occurred in a parallel fashion with the inhibition of acid secretion. No further increase in serum gastrin occurred with continued omeprazole administration. Increased gastrin causes enterochromaffin-like cell hyperplasia and increased serum Chromogranin A (CgA) levels. The increased CgA levels may lead to false positive results in diagnostic studies for neuroendocrine tumors. **Enterochromaffin-like (ECL) cell effects** Human gastric biopsy samples have been obtained from more than 3000 pediatric and adult patients treated with omeprazole in long-term clinical studies. The incidence of enterochromaffin-like cell hyperplasia in these studies increased with time; however, no case of ECL cell carcinoids, dysplasia, or neoplasia have been identified in these patients. These studies, however, are of insufficient in power and duration to draw conclusions on the possible influence of long-term administration of omeprazole in the development of any premalignant or malignant conditions. **Other effects** Systemic effects of omeprazole in the central nervous system, cardiovascular and respiratory systems have not been found to date. Omeprazole, given in oral doses of 30 or 40 mg for 2-4 weeks, showed no effect on thyroid function, carbohydrate metabolism, or circulating levels of parathyroid hormone, cortisol, estradiol, testosterone, prolactin, cholecystokinin or secretin. |
| 分子式 |
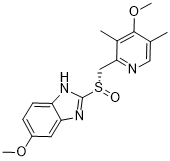
C17H19N3O3S
|
|---|---|
| 分子量 |
345.417
|
| 精确质量 |
345.114
|
| CAS号 |
119141-88-7
|
| 相关CAS号 |
Esomeprazole magnesium trihydrate;217087-09-7;Esomeprazole sodium;161796-78-7;Esomeprazole magnesium;161973-10-0;Esomeprazole magnesium salt;1198768-91-0;Esomeprazole potassium salt;161796-84-5;Esomeprazole hemistrontium;914613-86-8;Esomeprazole-d3 sodium;Esomeprazole-d3
|
| PubChem CID |
4594
|
| 外观&性状 |
Crystals from acetonitrile
White to off-white crystalline powder |
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
600.0±60.0 °C at 760 mmHg
|
| 闪点 |
316.7±32.9 °C
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
| 折射率 |
1.669
|
| LogP |
2.17
|
| tPSA |
96.31
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
453
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=[S@](C1=NC2=CC=C(OC)C=C2N1)CC3=NC=C(C)C(OC)=C3C
|
| InChi Key |
SUBDBMMJDZJVOS-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C17H19N3O3S/c1-10-8-18-15(11(2)16(10)23-4)9-24(21)17-19-13-6-5-12(22-3)7-14(13)20-17/h5-8H,9H2,1-4H3,(H,19,20)
|
| 化学名 |
1H-Benzimidazole, 5-methoxy-2-((S)-((4-methoxy-3,5-dimethyl-2- pyridinyl)methyl)sulfinyl)-
|
| 别名 |
Esomeprazole (–) Omeprazole(–)-Omeprazole (S) Omeprazole (S)-Omeprazole
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~125 mg/mL (~361.88 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8950 mL | 14.4751 mL | 28.9503 mL | |
| 5 mM | 0.5790 mL | 2.8950 mL | 5.7901 mL | |
| 10 mM | 0.2895 mL | 1.4475 mL | 2.8950 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
An Open-label, DDI Study to Investigate the Effects of Amlitelimab on the PK of Selected Cytochrome P450 Substrates
CTID: NCT06686628
Phase: Phase 1 Status: Recruiting
Date: 2024-11-13
|
|
|