| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Ferroptosis (EC50 = 60 nM)
Ferrostatin-1 (Fer-1) targets ferroptosis, a non-apoptotic cell death pathway driven by iron-dependent lipid peroxidation. It inhibits lipid radical propagation, with an EC50 of 60 nM for protecting HT-1080 cells from erastin-induced ferroptosis [2] - Ferrostatin-1 (Fer-1) exhibits antifungal activity by inhibiting fungal lipid peroxidation, with minimum inhibitory concentrations (MICs) of 2 μg/mL against Candida albicans and 4 μg/mL against Aspergillus fumigatus [3] |
|---|---|
| 体外研究 (In Vitro) |
Ferrostatin-1 抑制由erastin 产生的脂质和胞质ROS 的积累。在器官型大鼠脑切片中,ferrostatin-1 可抑制谷氨酸引起的神经毒性 [1]。 Ferrostatin-1(2 μM;24 小时)可保护大鼠器官型海马切片培养物 (OHSC) 免受谷氨酸 (5 mM) 诱导的神经毒性 [2]。 Ferrostatin-1 抑制脂质过氧化,但不抑制溶酶体膜的通透性或线粒体中活性氧的产生 [2]。在肾衰竭、脑室周围白质软化 (PVL) 和亨廷顿病 (HD) 的细胞模型中,ferrostatin-1 可减少细胞死亡 [2]。在 HT-1080 细胞中,ferrostatin-1(1 μM;6 小时)可防止不饱和脂肪酸氧化降解,从而增加健康中型多棘神经元 (MSN) 的数量 [3]。
HT-1080纤维肉瘤细胞铁死亡抑制:erastin(10 μM,铁死亡诱导剂)处理前1小时加入Fer-1(10-1000 nM),可显著提高细胞活力。60 nM时,细胞活力从erastin单独处理组的22%升至85%(MTT法);C11-BODIPY染色显示,100 nM Fer-1使erastin诱导的脂质过氧化减少72%(流式细胞术检测)[2] - 小鼠胚胎成纤维细胞(MEF)实验:Fer-1(50-500 nM)保护MEF免受RSL3(GPX4抑制剂)诱导的铁死亡。200 nM时,丙二醛(MDA,脂质过氧化标志物)水平降低65%(TBARS法),谷胱甘肽(GSH)水平得以维持(仅降低30%,而RSL3单独组降低75%)[2] - 抗真菌活性:Fer-1(0.5-16 μg/mL)抑制病原真菌生长。对白色念珠菌(ATCC 90028)的MIC为2 μg/mL,烟曲霉(ATCC 204305)为4 μg/mL,新型隐球菌(ATCC 24067)为8 μg/mL;4 μg/mL Fer-1使白色念珠菌的MDA水平降低58%[3] - LPS诱导BEAS-2B(人支气管上皮细胞)损伤:Fer-1(1-10 μM)预处理减少LPS(1 μg/mL)诱导的细胞死亡。5 μM时,细胞活力从52%升至83%,TNF-α mRNA表达降低62%(qPCR),脂质活性氧(ROS)水平降低55%(DCFH-DA染色)[4] - 缺氧缺血(HI)诱导新生大鼠原代皮质神经元损伤:Fer-1(0.1-1 μM)提高神经元活力。0.5 μM时,乳酸脱氢酶(LDH)释放减少48%(LDH法),GPX4蛋白表达升高2.1倍(Western blot)[6] |
| 体内研究 (In Vivo) |
在横纹肌溶解症小鼠中,Ferrostatin-1(5 mg/kg;腹膜内注射;单剂量,注射甘油前 30 分钟给药)可改善肾功能;然而,这种益处在缺乏泛半胱天冬酶抑制剂 zVAD 或 RIPK3 的小鼠中并未显示出来。 Frostatin-1(0.8 mg/kg;尾静脉注射)可以有效治疗 LPS 引起的急性肺损伤(ALI)[4]。 Ferrostatin-1(5 mg/kg;腹腔注射;C57BL/6J 小鼠)可改善横纹肌溶解症小鼠的肾功能 [5]。
研究结果显示,Ferrostatin-1(Fer-1)显著减轻了由缺氧和缺血引发的脑损伤。Fer-1显著增加SLC3A2、SLC7A11、ACSL3、GSS和GPX4的表达(P<0.05),显著降低GFAP、ACSL4、TFRC、FHC、FLC、4-HNE、HIF-1α和ROS的表达(P<0.05)。 结论:Fer-1通过潜在靶向GPX4/ACL3/ACSL4轴抑制铁下垂并缓解HIBD;然而,其具体机制值得进一步探索。[6] 绝经后口干症的机制尚未完全阐明。本研究旨在探讨绝经后动物模型中口干症的机制以及铁蛋白抑制剂去铁胺(DFO)和Ferrostatin-1(FER)对唾液腺功能障碍的影响。24只雌性Sprague-Dawley大鼠随机分为四组:SHAM组(n=6,假手术大鼠)、OVX组(n=5,去卵巢大鼠),FER组(n=7,切除卵巢大鼠腹腔注射FER)和DFO组(n=8,切除卵巢的大鼠腹腔内注射DFO)。分析GPX4活性、铁积累、脂质过氧化、炎症、纤维化和唾液腺功能。DFO组GPX4活性恢复,铁积累和细胞质MDA+HAE减少。此外,与OVX组相比,DFO组的I型胶原、III型胶原、TGF-β、IL-6、TNF-α和TGF-β水平降低。FER组还观察到GPX4活性和线粒体形态的恢复,以及细胞质MDA+HAE的减少。此外,在DFO和FER组中观察到炎性细胞因子和纤维化标志物的表达降低,AQP5的表达增加。绝经后唾液腺功能障碍与铁下垂有关,DFO和FER可能逆转绝经后的唾液腺功能障碍。因此,DFO和FER被认为是治疗绝经后口干症的有前景的方法。[7] 小鼠LPS诱导急性肺损伤(ALI)模型:6-8周龄雄性C57BL/6小鼠气管滴注LPS(5 mg/kg)诱导ALI,LPS处理前1小时腹腔注射Fer-1(5 mg/kg)可显著缓解损伤:肺湿重/干重比(水肿指标)从5.8降至3.2,支气管肺泡灌洗液(BALF)中TNF-α和IL-6水平分别降低55%和60%,肺组织MDA水平降低48%;组织病理学显示肺泡出血和炎症细胞浸润减少[4] - 新生大鼠缺氧缺血性脑损伤(HIBD)模型:7日龄(P7)SD大鼠行右侧颈总动脉结扎+2小时缺氧(8% O₂)处理,缺氧后立即腹腔注射Fer-1(10 mg/kg),72小时后脑梗死体积减少42%(TTC染色);神经行为评分改善(转棒实验:120秒 vs. 65秒;握力:假手术组的85% vs. 52%),脑脂质ROS水平降低58%[6] - 去卵巢(OVX)大鼠唾液腺功能障碍模型:8周龄雌性SD大鼠行双侧卵巢切除术,Fer-1(5 mg/kg/天,皮下注射)处理4周,唾液流量增加45%(从0.5 mL/10分钟升至0.72 mL/10分钟),唾液腺MDA水平降低52%,超氧化物歧化酶(SOD)活性升高2.3倍;组织病理学显示腺泡细胞结构保留(萎缩率从40%降至15%)[7] |
| 酶活实验 |
蛋白质印迹[4]
在我们的研究中,使用放射免疫沉淀分析裂解缓冲液裂解细胞样品,并使用Pierce BCA蛋白质分析试剂盒检测不同组的总蛋白质浓度。在我们的研究中,细胞裂解物(20 μg/泳道)用10%SDS-PAGE凝胶分离,然后转移到硝化纤维膜上。用在PBS中稀释的5%脱脂奶粉封闭膜,并进一步与一级抗体在4 °C。在此,使用的不同一级抗体是:抗SLC7A11(1:3000;细胞信号,类别号:12691)、抗GPX4(1:1000)、抗FTH(1:2000)和抗GAPDH(1:3000)。所用的第二抗体是:抗小鼠IgG(HRP缀合;1:5000)和抗兔IgG(HRP-缀合;1:10000)。最后,使用SuperSignal West Femto Maximum Sensitivity Substrate和ChemiDoc Images对每条泳道中的蛋白质带进行可视化。最后使用ImageJ1.x软件对结果进行量化。整个论文中图像的所有未切割的原始印迹如补充图所示。1。[4] 丙二醛(MDA)、4-羟基壬烯醛(4-HNE)和铁水平的评估[4] 在我们的研究中,为了评估不同组的脱铁水平,检测各组的MDA、4-HNE和铁水平。细胞裂解物中的MDA浓度、4-HNE浓度和铁浓度根据制造商的说明使用脂质过氧化(MDA)测定试剂盒、脂质过氧化测定试剂盒和铁测定试剂盒进行评估。 脂质过氧化抑制实验(TBARS法):取100 μL细胞或组织匀浆,与200 μL硫代巴比妥酸(TBA)试剂(0.67% TBA溶于50%冰醋酸)混合,95°C加热30分钟,冰浴冷却后3000×g离心10分钟,检测上清液532 nm吸光度。以1,1,3,3-四甲氧基丙烷为标准品制作标准曲线,计算MDA浓度。实验中在加入TBA试剂前加入Fer-1(100 nM-10 μM),评估其对脂质过氧化的抑制作用[2,4,6] - GPX4活性实验:取50 μg组织匀浆蛋白,与反应缓冲液(50 mM Tris-HCl pH 7.6、1 mM GSH、0.2 mM H₂O₂、0.1 mM NADPH)混合,检测340 nm吸光度变化(反映NADPH氧化)5分钟,GPX4活性以每毫克蛋白每分钟氧化的NADPH纳摩尔数计算。实验中Fer-1(0.5-5 μM)与匀浆预孵育30分钟,评估其对GPX4活性的影响[6] |
| 细胞实验 |
细胞活力测定[4]
为了评估细胞活力,我们的研究中使用CCK-8方法作为参考。简而言之,将BEAS-2B细胞以5的浓度接种到96孔板中 × 104个细胞/孔。细胞培养24小时 h、 然后用不同浓度的LPS和Fer-1处理16 h,然后添加20 μl CCK-8溶液直接加入培养基(200 μl/孔)并在37 °C 4 h.在450时检测不同组的吸光度(Abs) nm(n= 3.在空白组中,孔仅包含培养基,未经任何处理的细胞用作对照组。这里,细胞活力 = (实验组Abs空白组Abs)/(对照组Abs × 100%. HT-1080细胞铁死亡保护实验:HT-1080细胞以5×10³细胞/孔接种于96孔板,含10% FBS的DMEM培养24小时,erastin(10 μM)处理前1小时加入Fer-1(10-1000 nM)。24小时后加入20 μL MTT(5 mg/mL),孵育4小时后DMSO溶解甲瓒,检测570 nm吸光度计算细胞活力[2] - C11-BODIPY脂质过氧化染色:6孔板中2×10⁵细胞/孔的HT-1080细胞,用Fer-1(100 nM)+erastin(10 μM)处理12小时,37°C下5 μM C11-BODIPY(脂质ROS探针)染色30分钟,PBS洗涤后流式细胞术分析(激发488 nm,非氧化探针发射515 nm,氧化探针发射580 nm)[2] - 真菌MIC实验:真菌在含2%葡萄糖的RPMI 1640培养基中培养至对数期,96孔板中加入Fer-1(0.5-16 μg/mL),再加入真菌悬液(1×10⁴ CFU/孔),35°C孵育(念珠菌24-48小时,曲霉菌72小时)。MIC定义为抑制≥90%真菌生长的最低Fer-1浓度(600 nm吸光度检测)[3] - 新生大鼠原代皮质神经元HI损伤实验:从P1-P3 SD大鼠分离皮质神经元,含B27添加剂的Neurobasal培养基培养7天,氧糖剥夺(OGD:1% O₂、无糖培养基)2小时后复氧,复氧时加入Fer-1(0.1-1 μM)。24小时后LDH法检测LDH释放,Western blot检测GPX4蛋白[6] |
| 动物实验 |
Animal/Disease Models: Male C57BL/6 mice (LPS-induced ALI)[4]
Doses: 0.8 mg/kg Route of Administration: Tail vein injection Experimental Results: Exerted therapeutic action against LPS-induced ALI. In our study, the male C57BL/6 mice were divided randomly into 4 groups (n = 4 per group, 8–10 weeks old, weight = 23–25 g): the control group receiving 0.9% NaCl (containing 0.1% DMSO), the LPS group receiving LPS plus 0.9% NaCl (containing 0.1% DMSO), the Fer-1 group receiving Fer-1 only, and the LPS + Fer-1 group receiving both Fer-1 and LPS. The LPS-induced ALI model was induced by instilling intratracheally 50 μl of LPS solution (0.2 g/L), then Fer-1 (0.8 mg/kg) was administered after LPS challenge via tail vein injection. The Fer-1 was dissolved in DMSO first, and diluted with 0.9% NaCl. The final concentration of Fer-1 and DMSO was 0.2 mg/ml and 0.1% respectively. After the treatments for 16 h, the mice in each group were euthanized and bronchoalveolar lavage (BAL) fluid was collected via lung lavage. To analyze the differential BAL cell counts, the cells were concentrated using a Cytospin 4. Cell staining was performed using the Shandon Kwik-Diff kit. Additionally, the total protein concentration and the levels of IL-6 and TNF-α in each sample were detected with the Pierce BCA Protein Assay Kit, IL-6 ELISA Kit ELISA kit and TNF-α ELISA Kit according to the manufacturer’s instructions. Lung tissues in different groups were collected for qPCR and western blot detection, and part of lung tissues was fixed using 10% buffered formalin, then the tissues were embedded in paraffin for histological analyses as the references. Herein, a scoring system of 0–4 was used for the evaluation of lung injury as the reference. Mouse LPS-Induced ALI Model: Male C57BL/6 mice (6-8 weeks old, 20-22 g) were housed under SPF conditions (22±2°C, 12-hour light/dark cycle). Mice were randomized into 3 groups (n=8/group): 1. Sham: Intratracheal instillation of saline + intraperitoneal injection of saline (10 mL/kg); 2. LPS-only: Intratracheal instillation of LPS (5 mg/kg, dissolved in saline) + intraperitoneal saline; 3. LPS+Fer-1: Intraperitoneal injection of Fer-1 (5 mg/kg, dissolved in 0.1% DMSO + saline) 1 hour before LPS. Twenty-four hours after LPS instillation, mice were euthanized. Lungs were excised: one lobe was used to measure wet/dry weight ratio, another was homogenized for MDA assay, and BALF was collected to detect TNF-α/IL-6 levels (ELISA) [4] - Neonatal Rat HIBD Model: P7 Sprague-Dawley rats (10-12 g) were anesthetized with isoflurane. The right common carotid artery was ligated with 6-0 silk suture, then rats were placed in a hypoxia chamber (8% O₂, 92% N₂) for 2 hours. Rats were randomized into 3 groups (n=10/group): 1. Sham: Sham ligation + normoxia + intraperitoneal saline; 2. HI-only: Ligation + hypoxia + intraperitoneal saline; 3. HI+Fer-1: Intraperitoneal injection of Fer-1 (10 mg/kg, dissolved in 0.1% DMSO + saline) immediately after hypoxia. Seventy-two hours post-HI, rats were euthanized: brains were used for TTC staining (infarction volume) and lipid ROS assay; neurobehavioral tests (rotarod, grip strength) were performed 24 hours before euthanasia [6] - OVX Rat Salivary Gland Dysfunction Model: Female Sprague-Dawley rats (8 weeks old, 220-250 g) were anesthetized with pentobarbital sodium. Bilateral ovariectomy was performed (sham group: only laparotomy). Rats were randomized into 3 groups (n=6/group): 1. Sham: Sham operation + subcutaneous injection of saline; 2. OVX-only: Ovariectomy + subcutaneous saline; 3. OVX+Fer-1: Ovariectomy + subcutaneous injection of Fer-1 (5 mg/kg/day, dissolved in 0.1% DMSO + saline). After 4 weeks, salivary flow rate was measured (stimulated by pilocarpine, 5 mg/kg intraperitoneal). Salivary glands were excised: one part was homogenized for MDA/SOD assay, another was fixed in 4% paraformaldehyde for histopathology (H&E staining) [7] |
| 毒性/毒理 (Toxicokinetics/TK) |
Acute in vitro toxicity: Fer-1 (0.1-10 μM) treatment for 48 hours in HT-1080, MEF, and BEAS-2B cells showed no cytotoxicity—cell viability remained >90% (MTT/CCK-8 assay) [2,4]
- Acute in vivo toxicity: Mice/rats treated with Fer-1 (5-10 mg/kg, intraperitoneal/subcutaneous) for 1-4 weeks showed no abnormal behavior (e.g., lethargy, diarrhea), weight loss (<5% of baseline), or changes in serum ALT, AST, BUN, or creatinine levels. Histopathological examination of liver, kidney, and target organs (lung, brain, salivary gland) showed no tissue damage [4,6,7] |
| 参考文献 | |
| 其他信息 |
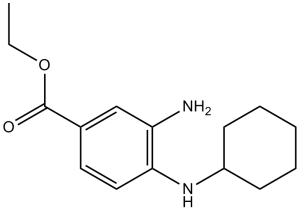
Ferrostatin-1 is an ethyl ester resulting from the formal condensation of the carboxy group of 3-amino-4-(cyclohexylamino)benzoic acid with ethanol. It is a potent inhibitor of ferroptosis, a distinct non-apoptotic form of cell death caused by lipid peroxidation. It is also a radical-trapping antioxidant and has the ability to reduce the accumulation of lipid peroxides and chain-carrying peroxyl radicals. It has a role as a ferroptosis inhibitor, a radiation protective agent, an antioxidant, a radical scavenger, an antifungal agent and a neuroprotective agent. It is a substituted aniline, an ethyl ester and a primary arylamine.
Background: Ferroptosis is a newly recognized type of cell death, which is different from traditional necrosis, apoptosis or autophagic cell death. However, the position of ferroptosis in lipopolysaccharide (LPS)-induced acute lung injury (ALI) has not been explored intensively so far. In this study, we mainly analyzed the relationship between ferroptosis and LPS-induced ALI. Methods: In this study, a human bronchial epithelial cell line, BEAS-2B, was treated with LPS and ferrostatin-1 (Fer-1, ferroptosis inhibitor). The cell viability was measured using CCK-8. Additionally, the levels of malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), and iron, as well as the protein level of SLC7A11 and GPX4, were measured in different groups. To further confirm the in vitro results, an ALI model was induced by LPS in mice, and the therapeutic action of Fer-1 and ferroptosis level in lung tissues were evaluated. Results: The cell viability of BEAS-2B was down-regulated by LPS treatment, together with the ferroptosis markers SLC7A11 and GPX4, while the levels of MDA, 4-HNE and total iron were increased by LPS treatment in a dose-dependent manner, which could be rescued by Fer-1. The results of the in vivo experiment also indicated that Fer-1 exerted therapeutic action against LPS-induced ALI, and down-regulated the ferroptosis level in lung tissues. Conclusions: Our study indicated that ferroptosis has an important role in the progression of LPS-induced ALI, and ferroptosis may become a novel target in the treatment of ALI patients.[4] Background: Hypoxic-ischemic brain damage (HIBD) is a type of brain damage that is caused by perinatal asphyxia and serious damages the central nervous system. At present, there is no effective drug for the treatment of this disease. Besides, the pathogenesis of HIBD remains elusive. While studies have shown that ferroptosis plays an important role in HIBD, its role and mechanism in HIBD are yet to be fully understood. Methods: The HIBD model of neonatal rats was established using the Rice-Vannucci method. A complete medium of PC12 cells was adjusted to a low-sugar medium, and the oxygen-glucose deprivation model was established after continuous hypoxia for 12 h. Laser Doppler blood flow imaging was used to detect the blood flow intensity after modeling. 2,3,5-triphenyl tetrazolium chloride staining was employed to detect ischemic cerebral infarction in rat brain tissue, and hematoxylin and eosin staining and transmission electron microscopy were used to observe brain injury and mitochondrial damage. Immunofluorescence was applied to monitor the expression of GFAP. Real-time quantitative polymerase chain reaction, western blot, and immunofluorescence were utilized to detect the expression of messenger RNA and protein. The level of reactive oxygen species (ROS) in cells was detected using the ROS detection kit. Results: The results showed that ferrostatin-1 (Fer-1) significantly alleviated the brain injury caused by hypoxia and ischemia. Fer-1 significantly increased the expression of SLC3A2, SLC7A11, ACSL3, GSS, and GPX4 (P<0.05) and dramatically decreased the expressions of GFAP, ACSL4, TFRC, FHC, FLC, 4-HNE, HIF-1α, and ROS (P<0.05). Conclusions: Fer-1 inhibits ferroptosis and alleviates HIBD by potentially targeting the GPX4/ACSL3/ACSL4 axis; however, its specific mechanism warrants further exploration.[6] The mechanism underlying xerostomia after menopause has not yet been fully elucidated. This study aimed to investigate the mechanism of xerostomia and the effect of the ferroptosis inhibitors deferoxamine (DFO) and ferrostatin-1 (FER) on salivary gland dysfunction in a postmenopausal animal model. Twenty-four female Sprague-Dawley rats were randomly divided into four groups: a SHAM group (n = 6, sham-operated rats), an OVX group (n = 6, ovariectomized rats), an FER group (n = 6, ovariectomized rats injected intraperitoneally with FER), and a DFO group (n = 6, ovariectomized rats injected intraperitoneally with DFO). GPX4 activity, iron accumulation, lipid peroxidation, inflammation, fibrosis, and salivary gland function were analyzed. Recovery of GPX4 activity and a decrease in iron accumulation and cytosolic MDA + HAE were observed in the DFO group. In addition, collagen I, collagen III, TGF-β, IL-6, TNF-α, and TGF-β levels were decreased in the DFO group compared to the OVX group. Recovery of GPX4 activity and the morphology of mitochondria, and reduction of cytosolic MDA + HAE were also observed in the FER group. In addition, decreased expression of inflammatory cytokines and fibrosis markers and increased expression of AQP5 were observed in both the DFO and FER groups. Postmenopausal salivary gland dysfunction is associated with ferroptosis, and DFO and FER may reverse the postmenopausal salivary gland dysfunction after menopause. DFO and FER are hence considered promising treatments for postmenopausal xerostomia.[7] Mechanism of action: Ferrostatin-1 (Fer-1) inhibits ferroptosis by scavenging lipid peroxyl radicals (LOO•) and preventing iron-dependent lipid peroxidation chain reactions. It does not directly chelate iron or activate GPX4 but preserves GPX4 function by reducing lipid ROS levels [2,4,6] - Research applications: Fer-1 is a widely used tool compound to study ferroptosis in vitro and in vivo. It has been applied to investigate ferroptosis-related diseases, including acute organ injury (lung, brain), neurodegenerative diseases, and cancer (as a potential chemoprotective agent for normal cells) [2,4,6] - Antifungal mechanism: Unlike traditional antifungals (e.g., azoles), Fer-1 targets fungal lipid metabolism by inhibiting lipid peroxidation, reducing fungal membrane integrity and growth. It shows synergistic activity with fluconazole against fluconazole-resistant C. albicans (FICI = 0.5) [3] - Limitations: Fer-1 has poor aqueous solubility (requires DMSO for dissolution) and is primarily used in preclinical research; it has not been evaluated in clinical trials or approved by the FDA for therapeutic use [2,4,6,7] |
| 分子式 |
C15H22N2O2
|
|
|---|---|---|
| 分子量 |
262.35
|
|
| 精确质量 |
262.168
|
|
| 元素分析 |
C, 68.67; H, 8.45; N, 10.68; O, 12.20
|
|
| CAS号 |
347174-05-4
|
|
| 相关CAS号 |
|
|
| PubChem CID |
4068248
|
|
| 外观&性状 |
Gray to gray purple solid
|
|
| 密度 |
1.1±0.1 g/cm3
|
|
| 沸点 |
437.3±35.0 °C at 760 mmHg
|
|
| 闪点 |
218.3±25.9 °C
|
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
|
| 折射率 |
1.595
|
|
| LogP |
3.9
|
|
| tPSA |
64.35
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
19
|
|
| 分子复杂度/Complexity |
290
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O(C([H])([H])C([H])([H])[H])C(C1C([H])=C([H])C(=C(C=1[H])N([H])[H])N([H])C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H])=O
|
|
| InChi Key |
UJHBVMHOBZBWMX-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C15H22N2O2/c1-2-19-15(18)11-8-9-14(13(16)10-11)17-12-6-4-3-5-7-12/h8-10,12,17H,2-7,16H2,1H3
|
|
| 化学名 |
3-amino-4-(cyclohexylamino)-benzoic acid, ethyl ester
|
|
| 别名 |
Frer-1; 3-amino-4-(cyclohexylamino)-benzoic acid, ethyl ester; Ferrostatin-1; 347174-05-4; Ethyl 3-amino-4-(cyclohexylamino)benzoate; Fer-1; Ferrostatin-1 (Fer-1); Ferrostatin 1; ferrrostatin 1; MFCD08072959;
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.53 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (9.53 mM) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (7.93 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 0.2 mg/mL (0.76 mM) in 10% DMSO + 90% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 5 中的溶解度: 2% DMSO+50% PEG 300+5% Tween 80+ddH2O: 5mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.8117 mL | 19.0585 mL | 38.1170 mL | |
| 5 mM | 0.7623 mL | 3.8117 mL | 7.6234 mL | |
| 10 mM | 0.3812 mL | 1.9059 mL | 3.8117 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|