| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Angiotensin-converting enzyme (ACE); [1][2][3]
|
|---|---|
| 体外研究 (In Vitro) |
福辛普利部分抑制脂质体和重组 LPLA2 的共沉降(0、1、10、33、100 μM;30 分钟)[1]。 LPLA2 的可溶性酯酶活性不受福辛普利 (250 nM) 的抑制 [1]。福辛普利(0.372、0.744、1.116 μM)的 Ki 值为 1.675 μM,以非竞争性方式抑制 ACE 活性 [2]。
|
| 体内研究 (In Vivo) |
通过降低乳酸脱氢酶 (LDH) 和肌酸激酶 (CK) 的水平,福辛普利(口服;4.67 mg/kg;4 周)可以抵消心脏功能障碍和结构改变 [3]。在急性心肌梗死大鼠模型中,福辛普利(口服;4.67 mg/kg;4 周)可减少 cleaved-caspase 3 的表达和心肌细胞凋亡。
在纽约心脏协会(NYHA)心功能II–III级的心力衰竭患者中,口服Fosinopril Sodium (SQ28555)(10 mg,每日1次)持续12周,显著改善血流动力学参数: 1. 收缩压(SBP)从基线的135 ± 12 mmHg降至118 ± 10 mmHg,舒张压(DBP)从82 ± 8 mmHg降至72 ± 6 mmHg。 2. 肺毛细血管楔压(PCWP,左心室充盈压指标)从22 ± 4 mmHg降至15 ± 3 mmHg。 3. 心输出量(CO)从3.2 ± 0.5 L/min升至4.1 ± 0.6 L/min,每搏输出量(SV)从45 ± 6 mL/次升至58 ± 5 mL/次。 4. 体循环血管阻力(SVR)降低28% ± 4%(从1850 ± 200 dyn·s/cm⁵降至1330 ± 150 dyn·s/cm⁵)[3] |
| 动物实验 |
Animal/Disease Models: Acute myocardial infarction (AMI) rat model after heart failure (SPF grade SD (SD (Sprague-Dawley)) rat, 265±15g) [3]
Doses: 4.67mg/kg Route of Administration: oral; 4-week Experimental Results: Combats cardiac dysfunction and structural changes and inhibits apoptosis. |
| 药代性质 (ADME/PK) |
Absorption: The oral bioavailability of Fosinopril Sodium (SQ28555) (a prodrug) in healthy volunteers was approximately 36%, and food intake did not significantly affect its absorption (AUC and Cmax changed by <10%). Peak plasma concentration of its active metabolite fosinoprilat was reached 3 hours after oral administration [2]
- Distribution: The volume of distribution (Vd) of fosinoprilat (active metabolite) was approximately 13 L in healthy volunteers. Fosinopril Sodium (SQ28555) and fosinoprilat did not penetrate the blood-brain barrier [2] - Metabolism: Fosinopril Sodium (SQ28555) was rapidly metabolized in the liver and gastrointestinal tract via esterase hydrolysis to form its active metabolite fosinoprilat (the main form exerting ACE inhibitory activity). No other active metabolites were detected [2] - Excretion: Fosinoprilat was excreted equally via the kidneys and bile (50% each) in healthy volunteers. This dual-excretion pathway reduced the risk of accumulation in patients with single-organ (renal or hepatic) impairment. The elimination half-life (t1/2) of fosinoprilat was approximately 11.5 hours [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Plasma protein binding: Fosinoprilat (active metabolite) had a plasma protein binding rate of approximately 95% [2]
- Adverse effects: The most common adverse effects of Fosinopril Sodium (SQ28555) in clinical use included dry cough (incidence: 4%–7%), dizziness (2%–3%), and fatigue (1%–2%). Rare adverse effects included angioedema (incidence <0.1%) and hyperkalemia (more common in patients with renal impairment or concurrent use of potassium supplements) [2] - Drug-drug interactions: Concomitant use with loop diuretics (e.g., furosemide) increased the risk of hypotension. Concomitant use with non-steroidal anti-inflammatory drugs (NSAIDs, e.g., ibuprofen) may reduce the antihypertensive and hemodynamic effects of Fosinopril Sodium (SQ28555) [2] |
| 参考文献 |
|
| 其他信息 |
Fosinopril sodium is the sodium salt of fosinopril. It is used for the treatment of hypertension and heart failure. A pro-drug, its phosphinate ester group is hydrolysed in vivo to give the corresponding phosphininc acid, fosinoprilat, which is the active metabolite. It has a role as an EC 3.4.15.1 (peptidyl-dipeptidase A) inhibitor, a prodrug and an antihypertensive agent. It contains a fosinopril(1-).
Fosinopril Sodium is the sodium salt of fosinopril, a phosphinic acid-containing angiotensin-converting enzyme (ACE) inhibitor with antihypertensive activity. Fosinopril sodium is an ester prodrug that is hydrolysed by esterases to its active metabolite fosinoprilat. Fosinoprilat specifically and competitively inhibits angiotensin-converting enzyme thereby decreasing the formation of the potent vasoconstrictor angiotensin II, resulting in diminished vasopressor activity. In addition, angiotensin II-mediated aldosterone secretion by adrenal cortex is decreased, which results in a decrease of sodium retention and an increase of serum potassium. (NCI05) A phosphinic acid-containing angiotensin-converting enzyme inhibitor that is effective in the treatment of hypertension. It is a prodrug that is converted to its active metabolite fosinoprilat. See also: Fosinopril (has active moiety); Fosinoprilat (has active moiety); Fosinopril sodium; hydrochlorothiazide (component of). 1. Fosinopril Sodium (SQ28555) is a prodrug angiotensin-converting enzyme inhibitor (ACEI) with a unique phosphinate structure. Unlike other ACEIs (e.g., captopril, enalapril), it contains a phosphonate group instead of a sulfhydryl or carboxylate group, which contributes to its dual renal-biliary excretion profile [1][2] 2. Its pharmacological mechanism involves inhibiting ACE to reduce the conversion of angiotensin I to angiotensin II (a potent vasoconstrictor), thereby dilating systemic and pulmonary blood vessels and improving cardiac hemodynamics in heart failure [2][3] 3. Therapeutic indications: Fosinopril Sodium (SQ28555) is indicated for the treatment of essential hypertension (monotherapy or combination therapy) and heart failure (adjunctive therapy with diuretics and/or digoxin) [2] 4. In patients with renal impairment (creatinine clearance 10–40 mL/min) or hepatic impairment, no dose adjustment of Fosinopril Sodium (SQ28555) is required due to its dual-excretion pathway, which is an advantage over ACEIs excreted solely via the kidneys [2] |
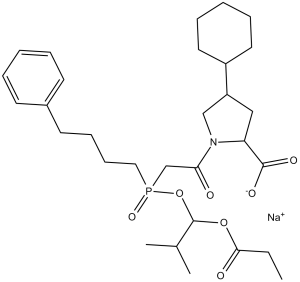
| 分子式 |
C30H45NO7P.NA
|
|---|---|
| 分子量 |
585.64
|
| 精确质量 |
563.301
|
| CAS号 |
88889-14-9
|
| 相关CAS号 |
Fosinopril;98048-97-6
|
| PubChem CID |
23681451
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
705.7±70.0 °C at 760 mmHg
|
| 熔点 |
196-198ºC
|
| 闪点 |
380.6±35.7 °C
|
| 蒸汽压 |
0.0±2.4 mmHg at 25°C
|
| 折射率 |
1.532
|
| LogP |
6.09
|
| tPSA |
122.85
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
15
|
| 重原子数目 |
40
|
| 分子复杂度/Complexity |
857
|
| 定义原子立体中心数目 |
2
|
| SMILES |
CCC(=O)OC(C(C)C)OP(=O)(CCCCC1=CC=CC=C1)CC(=O)N2C[C@@H](C[C@H]2C(=O)[O-])C3CCCCC3.[Na+]
|
| InChi Key |
TVTJZMHAIQQZTL-HREVRLCXSA-M
|
| InChi Code |
InChI=1S/C30H46NO7P.Na/c1-4-28(33)37-30(22(2)3)38-39(36,18-12-11-15-23-13-7-5-8-14-23)21-27(32)31-20-25(19-26(31)29(34)35)24-16-9-6-10-17-24;/h5,7-8,13-14,22,24-26,30H,4,6,9-12,15-21H2,1-3H3,(H,34,35);/q;+1/p-1/t25-,26+,30?,39?;/m1./s1
|
| 化学名 |
sodium;(2S,4S)-4-cyclohexyl-1-[2-[(2-methyl-1-propanoyloxypropoxy)-(4-phenylbutyl)phosphoryl]acetyl]pyrrolidine-2-carboxylate
|
| 别名 |
SQ-28,555;SQ 28,555; SQ 28555; SQ-28,555; SQ-28555; SQ28,555; SQ28555; Monopril; Staril; Dynacil; Fosinil; Tenso Stop; Tensocardil; Sodium, Fosinopril; Staril;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO:<1 mg/mL
Water:117 mg/mL (199.8 mM)
Ethanol:4 mg/mL (6.8 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 9.09 mg/mL (15.52 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7075 mL | 8.5377 mL | 17.0753 mL | |
| 5 mM | 0.3415 mL | 1.7075 mL | 3.4151 mL | |
| 10 mM | 0.1708 mL | 0.8538 mL | 1.7075 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。