| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
The targets of Galeterone (TOK-001) include cytochrome P450 17A1 (CYP17A1) and androgen receptor (AR). For CYP17A1, the inhibition constant (Ki) for C17,20-lyase activity is 1.9 nM, and the Ki for 17α-hydroxylase activity is 36 nM [2]
; it inhibits the transcriptional activity of AR, with a half-maximal inhibitory concentration (EC50) of approximately 2 μM in the AR reporter gene assay using LNCaP cells [3] . |
|---|---|
| 体外研究 (In Vitro) |
Galeterone (TOK-001) 抑制 CYP17 裂合酶,IC50 为 47 nM [1]。 Galeterone (TOK-001) 既是 CYP17A1 抑制剂,又是雄激素受体拮抗剂,这些结合模式的相似性很可能是其双重作用机制的原因。CYP17A1 与阿比特龙和 Galeterone (TOK-001) 结合,吸光度降低402 nm 并在 424 nm 处增加,与氮与血红素铁的结合(II 型相互作用)一致,Kd <100 nM[2]。当 LNCaP 细胞在补充有活性炭剥离血清 (CSS, T<1 nM) 的培养基中培养,然后用浓度不断增加的 Galeterone (TOK-001) 处理时,AR 蛋白的稳态水平显着降低(高达 84 %(15 μM Galeterone)。在 LAPC-4 细胞中,浓度高于 1 μM 的阿比特龙醇比加莱特龙 (TOK-001) 更能降低 AR 表达。用 20 μM TOK-001 处理 LNCaP 细胞 24 小时,AR mRNA 水平降低 38% [3]。
1. 对CYP17A1酶活性的抑制作用:在重组人CYP17A1体外酶活性实验中,Galeterone(TOK-001)对C17,20-裂合酶活性的IC50为2.5 nM,对17α-羟化酶活性的IC50为46 nM,可显著抑制雄激素合成通路中的关键酶活性[1] 2. 对前列腺癌细胞AR活性的调节作用:在LNCaP前列腺癌细胞中,Galeterone(TOK-001)(1-10 μM)处理可显著降低AR靶基因(如前列腺特异性抗原PSA、TMPRSS2)的mRNA表达水平(通过RT-PCR检测,与对照组相比下降50%-80%);同时通过Western blot检测发现,AR蛋白表达水平呈剂量依赖性降低[3] 3. 对前列腺癌细胞增殖的抑制作用:在LNCaP、C4-2B(AR依赖性前列腺癌细胞)中,Galeterone(TOK-001)处理72小时后的细胞增殖抑制IC50分别为1.8 μM和2.3 μM;而对PC-3(AR阴性前列腺癌细胞)的增殖无显著抑制作用(IC50 > 20 μM),表明其抗增殖活性具有AR依赖性[3] 4. 诱导前列腺癌细胞凋亡:在LNCaP细胞中,Galeterone(TOK-001)(10 μM)处理48小时后,通过Annexin V-FITC/PI双染流式细胞术检测,凋亡细胞比例(早期+晚期凋亡)从对照组的3.2%升高至18.5%[3] 。 |
| 体内研究 (In Vivo) |
Galeterone (TOK-001) 以 0.15 mmol/kg 的剂量每天两次皮下注射给已注射 LAPC-4 肿瘤的小鼠。第 31 天,TOK-001 治疗组小鼠的平均肿瘤体积低于对照组 (p = 0.0001)。与对照组相比,给予加莱特酮(TOK-001)导致肿瘤发生率显着降低(p<0.0001)。切除后,与用对照和去势治疗的动物相比,用加莱特隆 (TOK-001) 治疗的动物的最终肿瘤重量表现出显着降低 (p<0.05)。
在LAPC-4人前列腺癌裸鼠异种移植模型中,Galeterone(TOK-001)口服给药显示出显著的抗瘤活性。具体而言,每日口服10 mg/kg Galeterone(TOK-001)连续28天,肿瘤生长抑制率(TGI)为45%;每日口服100 mg/kg时,TGI达到78%,且给药期间肿瘤体积持续缩小(从给药前的142 mm³降至实验结束时的89 mm³)。相比之下,同等剂量(100 mg/kg)的阿比特龙(abiraterone)口服给药的TGI为62%,肿瘤体积从138 mm³降至101 mm³。实验结束时,100 mg/kg Galeterone(TOK-001)组的肿瘤重量显著低于溶媒对照组(0.32 g vs 0.85 g,P < 0.01),且未观察到肿瘤复发迹象[1] 在该模型中,通过免疫组化检测肿瘤组织中Ki-67(细胞增殖标志物)的表达,结果显示100 mg/kg Galeterone(TOK-001)组的Ki-67阳性细胞比例(12%)显著低于溶媒对照组(35%)和100 mg/kg阿比特龙组(18%),表明Galeterone(TOK-001)在体内可有效抑制肿瘤细胞增殖[1] |
| 酶活实验 |
1. CYP17A1 C17,20-裂合酶活性测定:将重组人CYP17A1、NADPH-细胞色素P450还原酶、细胞色素b5与底物(如17α-羟孕烯醇酮)在缓冲液中混合,加入不同浓度的Galeterone(TOK-001)或溶媒对照,37℃孵育特定时间后,加入终止液终止反应。通过HPLC或LC-MS检测产物(如脱氢表雄酮DHEA)的生成量,计算不同药物浓度对酶活性的抑制率,再通过非线性回归分析获得IC50值[1]
2. CYP17A1结合实验(基于X射线晶体学):将纯化的人CYP17A1蛋白与Galeterone(TOK-001)在4℃下孵育过夜,形成蛋白-药物复合物。采用悬滴气相扩散法对复合物进行结晶,收集晶体的X射线衍射数据,解析CYP17A1-Galeterone(TOK-001)复合物的结构,分析Galeterone(TOK-001)与CYP17A1活性位点的结合模式及关键相互作用位点(如氢键、疏水作用),明确其抑制机制[2] 3. AR转录活性测定(报告基因实验):将AR响应性荧光素酶报告质粒(含AR结合元件)和海肾荧光素酶质粒(内参)转染至LNCaP细胞。转染24小时后,加入不同浓度的Galeterone(TOK-001)或溶媒对照,并加入双氢睾酮(DHT)激活AR。处理24小时后裂解细胞,采用双荧光素酶报告基因检测系统测定荧光素酶活性,计算相对荧光素酶活性(萤火虫荧光素酶活性/海肾荧光素酶活性),评估Galeterone(TOK-001)对AR转录活性的抑制作用[3] 。 |
| 细胞实验 |
1. 前列腺癌细胞增殖实验(MTT法):将LNCaP或C4-2B细胞以3×10³个/孔的密度接种于96孔板,培养24小时。向孔中加入不同浓度的Galeterone(TOK-001)(0.1 μM、1 μM、5 μM、10 μM、20 μM)或溶媒对照,继续培养72小时。孵育结束后,每孔加入MTT试剂,37℃孵育4小时,去除上清液后加入DMSO溶解甲臜结晶。使用酶标仪在特定波长下检测吸光度,细胞存活率按(药物处理组吸光度/对照组吸光度)×100%计算,通过剂量-效应曲线拟合获得IC50值[3]
2. AR及靶蛋白Western blot实验:将LNCaP细胞接种于6孔板,培养至70%-80%融合度。用不同浓度的Galeterone(TOK-001)(1 μM、5 μM、10 μM)或溶媒对照处理细胞24小时,采用含蛋白酶抑制剂的裂解液裂解细胞,通过蛋白定量试剂盒测定总蛋白浓度。将等量蛋白经SDS-PAGE分离后转移至PVDF膜,用封闭液封闭后,加入抗AR、抗PSA和抗β-actin(内参)一抗,4℃孵育过夜。洗涤后加入辣根过氧化物酶标记的二抗,室温孵育1小时,采用化学发光试剂显影蛋白条带,通过图像分析软件定量条带强度,评估AR和PSA的相对表达水平[3] 3. AR靶基因表达RT-PCR实验:用Galeterone(TOK-001)(5 μM、10 μM)或溶媒对照处理LNCaP细胞24小时,采用RNA提取试剂盒提取总RNA,通过分光光度计测定RNA浓度和纯度。用逆转录试剂盒将总RNA合成cDNA,使用PSA、TMPRSS2和GAPDH(内参)特异性引物进行PCR扩增。PCR产物经琼脂糖凝胶电泳分离后定量条带强度,按(靶基因条带强度/GAPDH条带强度)×100%计算PSA和TMPRSS2的相对mRNA表达水平[3] 。 |
| 动物实验 |
Dissolved in a 0.3% solution of hydroxypropyl cellulose in saline; 50 mg/kg; s.c. injection
Male severe combined immunodeficient (SCID) mice inoculated subcutaneously (s.c.) with LAPC4 cells 1. LAPC-4 human prostate cancer xenograft model establishment and drug administration: Male nude mice (or SCID mice) aged 6-8 weeks were used. LAPC-4 human prostate cancer cells were suspended in a mixture of culture medium and Matrigel, and 1×10⁷ cells were subcutaneously injected into the right back of each mouse. When the tumor volume reached approximately 100-150 mm³, the mice were randomly divided into 4 groups (n=6-8 per group): vehicle control group (0.5% methylcellulose + 0.1% Tween 80), low-dose Galeterone (TOK-001) group (10 mg/kg), high-dose Galeterone (TOK-001) group (100 mg/kg), and abiraterone group (100 mg/kg). All drugs were administered by oral gavage once a day for 21-28 consecutive days. During the experiment, the body weight and tumor volume of the mice were measured every 2-3 days. The tumor volume was calculated using the formula: V = length × width² / 2. At the end of the experiment, the mice were euthanized, and the tumors were excised and weighed. The tumor growth inhibition rate (TGI) was calculated as [1 - (tumor weight of drug-treated group / tumor weight of control group)] × 100% [1] 2. Immunohistochemical analysis of tumor tissues: The excised tumor tissues were fixed in formalin, embedded in paraffin, and cut into 4-μm-thick sections. The sections were deparaffinized with xylene and rehydrated with gradient ethanol. Antigen retrieval was performed by boiling the sections in a citrate buffer. The sections were blocked with a blocking buffer and then incubated with a primary antibody against Ki-67 overnight at 4°C. After washing, the sections were incubated with a biotin-conjugated secondary antibody and then with streptavidin-horseradish peroxidase. The sections were stained with 3,3'-diaminobenzidine (DAB) and counterstained with hematoxylin. The number of Ki-67-positive cells and the total number of cells in 5 random high-power fields (400×) were counted, and the proportion of Ki-67-positive cells was calculated [1] . |
| 药代性质 (ADME/PK) |
1. In vitro metabolic stability assay: Galeterone (TOK-001) was mixed with rat or human liver microsomes in a buffer containing NADPH, and the mixture was incubated at 37°C. Samples were collected at different time points (0, 15, 30, 60, 90 minutes) and mixed with a protein precipitation reagent to terminate the reaction. After centrifugation, the supernatant was analyzed by LC-MS/MS to determine the concentration of the remaining Galeterone (TOK-001). The in vitro half-life (t1/2) of the drug was calculated based on the concentration-time curve. The results showed that the in vitro metabolic half-life of Galeterone (TOK-001) was significantly longer than that of its precursor VN/124-1, indicating improved metabolic stability [1]
2. Rat pharmacokinetic study: Male Sprague-Dawley rats were fasted for 12 hours before administration. Galeterone (TOK-001) was administered to the rats at an oral dose of 10 mg/kg. Blood samples were collected from the orbital venous plexus at different time points (0.25, 0.5, 1, 2, 4, 6, 8, 12, 24 hours) after administration, and plasma was separated by centrifugation. The plasma concentration of Galeterone (TOK-001) was determined by LC-MS/MS. Pharmacokinetic parameters were calculated using pharmacokinetic software: the peak plasma concentration (Cmax) was 856 ng/mL, the time to reach Cmax (Tmax) was 1.2 hours, the area under the plasma concentration-time curve from 0 to 24 hours (AUC0-24h) was 3240 ng·h/mL, and the oral bioavailability was approximately 38% [1] 3. Plasma protein binding assay: Galeterone (TOK-001) was incubated with rat or human plasma at 37°C for 1 hour to reach equilibrium. The mixture was then transferred to an ultrafiltration tube and centrifuged to separate the free drug (in the filtrate) from the protein-bound drug (on the filter membrane). The concentration of free Galeterone (TOK-001) in the filtrate was determined by LC-MS/MS. The plasma protein binding rate was calculated as [1 - (free drug concentration / total drug concentration)] × 100%. The results showed that the human plasma protein binding rate of Galeterone (TOK-001) was greater than 95% [1] . |
| 毒性/毒理 (Toxicokinetics/TK) |
1. In vivo general toxicity observation: During the LAPC-4 xenograft model experiment, the body weight of mice in each group was monitored daily. The results showed that there was no significant decrease in body weight in the Galeterone (TOK-001)-treated groups (low dose and high dose) compared with the vehicle control group (the body weight change rate was within ±10%). No obvious toxic symptoms (such as reduced activity, rough fur, diarrhea, or lethargy) were observed in the Galeterone (TOK-001)-treated mice [1]
2. Hematological and biochemical parameter detection: At the end of the animal experiment, blood samples were collected from the mice to detect hematological parameters (e.g., white blood cell count, red blood cell count, platelet count) and serum biochemical parameters (e.g., alanine transaminase ALT, aspartate transaminase AST, creatinine CRE). The results showed that there were no significant differences in these parameters between the Galeterone (TOK-001)-treated groups and the vehicle control group, indicating that Galeterone (TOK-001) did not cause obvious hematotoxicity or hepatotoxicity/nephrotoxicity at the tested doses [1] . |
| 参考文献 |
|
| 其他信息 |
Galeterone is a 3-hydroxy steroid. It has a role as an androgen.
Galeterone has been used in trials studying the treatment of Prostate Cancer. Galeterone is an orally bioavailable small-molecule androgen receptor modulator and CYP17 lyase inhibitor with potential antiandrogen activity. Galeterone exhibits three distinct mechanisms of action: 1) as an androgen receptor antagonist, 2) as a CYP17 lyase inhibitor and 3) by decreasing overall androgen receptor levels in prostate cancer tumors, all of which may result in a decrease in androgen-dependent growth signaling. Localized to the endoplasmic reticulum (ER), the cytochrome P450 enzyme CYP17 (P450C17 or CYP17A1) exhibits both 17alpha-hydroxylase and 17,20-lyase activities, and plays a key role in the steroidogenic pathway that produces progestins, mineralocorticoids, glucocorticoids, androgens, and estrogens. 1. Galeterone (TOK-001) is a metabolically stable analog of VN/124-1, designed to address the poor metabolic stability of VN/124-1. Structural modifications (e.g., introduction of specific functional groups) were made to VN/124-1 to improve its resistance to hepatic metabolism, thereby enhancing its in vivo efficacy and pharmacokinetic properties [1] 2. The inhibitory mechanism of Galeterone (TOK-001) on CYP17A1 is related to its binding to the active site of the enzyme. X-ray crystallography showed that Galeterone (TOK-001) binds to the heme iron in the active site of CYP17A1 through its nitrogen-containing functional group, and forms hydrophobic interactions with surrounding amino acid residues (e.g., Leu360, Ile363), thereby blocking the binding of the substrate to the enzyme and inhibiting enzyme activity [2] 3. Galeterone (TOK-001) exerts antitumor effects through a dual mechanism: it not only inhibits CYP17A1 to reduce androgen synthesis (indirect anti-AR effect) but also directly binds to AR and inhibits its transcriptional activity, thereby suppressing the growth of AR-dependent prostate cancer cells. This dual mechanism makes it effective in prostate cancer models, even in cases where abiraterone may have limited efficacy [3] 4. In the LAPC-4 xenograft model, Galeterone (TOK-001) at a dose of 100 mg/kg showed superior antitumor efficacy compared to abiraterone at the same dose, as evidenced by a higher TGI and greater tumor volume reduction. This suggests that Galeterone (TOK-001) may be a potential alternative to abiraterone for the treatment of castration-resistant prostate cancer (CRPC) [1] . |
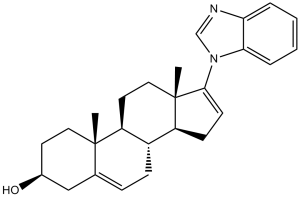
| 分子式 |
C26H32N2O
|
|
|---|---|---|
| 分子量 |
388.55
|
|
| 精确质量 |
388.251
|
|
| CAS号 |
851983-85-2
|
|
| 相关CAS号 |
|
|
| PubChem CID |
11188409
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
564.5±60.0 °C at 760 mmHg
|
|
| 熔点 |
189-190℃
|
|
| 闪点 |
295.2±32.9 °C
|
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
|
| 折射率 |
1.693
|
|
| LogP |
6.28
|
|
| tPSA |
38.05
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
2
|
|
| 可旋转键数目(RBC) |
1
|
|
| 重原子数目 |
29
|
|
| 分子复杂度/Complexity |
743
|
|
| 定义原子立体中心数目 |
6
|
|
| SMILES |
C[C@]12CC[C@@H](CC1=CC[C@@H]3[C@@H]2CC[C@]4([C@H]3CC=C4N5C=NC6=CC=CC=C65)C)O
|
|
| InChi Key |
PAFKTGFSEFKSQG-PAASFTFBSA-N
|
|
| InChi Code |
InChI=1S/C26H32N2O/c1-25-13-11-18(29)15-17(25)7-8-19-20-9-10-24(26(20,2)14-12-21(19)25)28-16-27-22-5-3-4-6-23(22)28/h3-7,10,16,18-21,29H,8-9,11-15H2,1-2H3/t18-,19-,20-,21-,25-,26-/m0/s1
|
|
| 化学名 |
(3S,8R,9S,10R,13S,14S)-17-(1H-benzo[d]imidazol-1-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol
|
|
| 别名 |
Galeterone; NX41765; NX 41765; TOK001; NX-41765; VN 1241; TOK-001; TOK 001; VN-1241; VN-1241
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.43 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.43 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.43 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 0.5% hydroxyethyl cellulose: 30 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5737 mL | 12.8684 mL | 25.7367 mL | |
| 5 mM | 0.5147 mL | 2.5737 mL | 5.1473 mL | |
| 10 mM | 0.2574 mL | 1.2868 mL | 2.5737 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02729376 | Completed | Drug: galeterone | Healthy | LTN PHARMACEUTICALS, INC. | March 2016 | Phase 1 |
| NCT04098081 | Recruiting | Drug: galeterone Drug: Gemcitabine |
Advanced Pancreatic Cancer | University of Maryland, Baltimore | December 12, 2019 | Phase 2 |
| NCT02438007 | Terminated | Drug: Galeterone Drug: Enzalutamide |
Prostate Cancer | LTN PHARMACEUTICALS, INC. | June 2015 | Phase 3 |
| NCT01709734 | Terminated | Drug: galeterone | Prostate Cancer | LTN PHARMACEUTICALS, INC | December 2012 | Phase 2 |
|
|---|
|
|