| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 靶点 |
Hedgehog (Hh) signaling pathway, specifically Smoothened (Smo) protein (IC50 ≈ 100 nM, determined by Smo-mediated Gli luciferase reporter gene activity in HEK293 cells) [1]
- Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) (IC50 ≈ 5 μM, measured by VEGFR2 kinase activity assay) and Phosphoinositide 3-Kinase (PI3K) (IC50 ≈ 2 μM, measured by PI3K kinase activity assay) [2] - Oxysterol-Binding Protein (OSBP) (IC50 ≈ 2 μM, determined by OSBP-cholesterol binding assay) and Hedgehog (Hh) signaling pathway (IC50 ≈ 150 nM for Gli reporter activity in PANC-1 cells) [3] |
|---|---|
| 体外研究 (In Vitro) |
伊曲康唑抑制 HUVEC 增殖(IC50 为 0.16 μM)[2]。在体外,伊曲康唑抑制内皮细胞周期的 G1 期[1]。
1. 抑制Hedgehog(Hh)通路及癌细胞增殖:在共转染Gli荧光素酶报告质粒、Smo表达质粒的HEK293细胞中,Itraconazole浓度依赖性抑制Smo介导的Gli转录活性,IC50约100 nM;200 nM时抑制率超80%。对Hh依赖性癌细胞系(如DAOY髓母细胞瘤细胞、MB031胶质母细胞瘤细胞),Itraconazole通过CCK-8法检测显示其增殖抑制IC50为50-200 nM。Western blot分析表明,100 nM Itraconazole处理DAOY细胞48小时后,Hh通路下游效应因子Gli1蛋白表达下调约60% [1] 2. 抗血管生成活性:Itraconazole抑制VEGF诱导的人脐静脉内皮细胞(HUVEC)增殖,MTT法测定IC50约3 μM。Transwell迁移实验中,10 μM Itraconazole处理24小时可减少60%的VEGF诱导HUVEC迁移;Matrigel管形成实验中,10 μM Itraconazole处理6小时可减少70%的HUVEC总管长。Western blot显示,5 μM Itraconazole可使HUVEC中VEGF诱导的VEGFR2磷酸化(p-VEGFR2)降低50%,PI3K磷酸化(p-PI3K)降低45% [2] 3. 抑制OSBP及与化疗药协同抗肿瘤:体外OSBP胆固醇转运实验中,2 μM Itraconazole抑制OSBP介导的膜间胆固醇转运达75%。与紫杉醇(化疗药)联用时,Itraconazole可增强A549肺癌细胞的增殖抑制活性:5 μM Itraconazole+10 nM紫杉醇处理72小时,增殖抑制率约85%,显著高于10 nM紫杉醇单药组(约45%)。对Hh依赖性PANC-1胰腺癌细胞,Itraconazole抑制Gli报告基因活性的IC50约150 nM,与文献[1]结果一致 [3] |
| 体内研究 (In Vivo) |
在小鼠同种异体移植模型中,替那唑治疗(75-100 mg/kg;口服灌胃;每天两次;持续 18 天;雌性远交无胸腺裸鼠)抑制髓母细胞瘤的生长和 Hh 通路活性[1]。
1. Hh依赖性肿瘤模型的抗肿瘤活性:4-6周龄裸鼠右侧皮下注射DAOY髓母细胞瘤细胞(5×10⁶个/只),待肿瘤平均体积达100 mm³时,随机分为2组(n=6/组):(a)对照组:口服0.5%羧甲基纤维素钠(CMC-Na);(b)Itraconazole组:口服50 mg/kg Itraconazole(溶解于0.5% CMC-Na),每日两次。处理21天后,Itraconazole组肿瘤平均体积为对照组的40%,平均肿瘤重量降低60%。肿瘤组织Western blot显示,Itraconazole组Gli1蛋白表达下调55% [1] 2. 体内模型的抗血管生成活性:(a)鸡胚绒毛尿囊膜(CAM)实验:鸡胚37℃孵育3天,蛋壳开窗后向CAM加入100 μg Itraconazole(溶解于DMSO),对照组加DMSO。孵育48小时后,Itraconazole组CAM血管密度较对照组降低50%。(b)HUVEC移植瘤模型:裸鼠皮下注射HUVEC(1×10⁷个/只),肿瘤达80 mm³时分为2组(n=6/组):对照组(腹腔注射生理盐水+5% DMSO)、Itraconazole组(20 mg/kg Itraconazole腹腔注射,每日1次)。14天后,Itraconazole组肿瘤体积为对照组的45% [2] 3. 与化疗药协同抗肿瘤活性:(a)A549肺癌移植瘤模型:裸鼠皮下注射A549细胞(2×10⁶个/只),肿瘤达120 mm³时分为4组(n=6/组):对照组、紫杉醇单药组(10 mg/kg,腹腔注射,每周1次)、Itraconazole单药组(100 mg/kg,口服,每日1次)、联合组(10 mg/kg紫杉醇+100 mg/kg Itraconazole)。30天后,联合组肿瘤体积为对照组的30%,显著低于紫杉醇单药组(对照组的55%)。(b)PANC-1胰腺癌模型:裸鼠接种PANC-1细胞(3×10⁶个/只),75 mg/kg Itraconazole口服(每日1次)处理28天后,肿瘤重量降低45% [3] |
| 酶活实验 |
1. Smo介导的Gli荧光素酶报告实验:(1)细胞转染:HEK293细胞以2×10⁴个/孔接种于96孔板,培养24小时后,用转染试剂共转染Gli-荧光素酶报告质粒(报告基因)、Smo表达质粒及Renilla荧光素酶质粒(内参)。(2)药物处理:转染24小时后,更换含不同浓度Itraconazole(0、10、50、100、200、500 nM)和100 nM Smo激动剂(SAG)的新鲜培养基。(3)检测:孵育24小时后裂解细胞,测定双荧光素酶活性,计算相对荧光活性(Gli-luc/Renilla-luc),确定Itraconazole抑制Smo的IC50 [1]
2. VEGFR2激酶活性实验:(1)反应体系制备:向96孔板中加入重组人VEGFR2激酶结构域(0.1 μg)、ATP(10 μM)、荧光标记底物肽及不同浓度Itraconazole(0、1、2、5、10、20 μM),总体积50 μL。(2)孵育:37℃孵育60分钟,允许激酶反应进行。(3)终止与检测:加入终止缓冲液终止反应,用酶标仪检测磷酸化底物肽的荧光强度,计算VEGFR2激酶活性抑制率并确定IC50 [2] 3. OSBP胆固醇结合实验:(1)蛋白制备:重组人OSBP蛋白(0.5 μg)溶解于结合缓冲液。(2)结合反应:在96孔黑色板中,将OSBP蛋白与荧光标记胆固醇类似物(200 nM)及不同浓度Itraconazole(0、0.5、1、2、5、10 μM)混合。(3)孵育与检测:4℃孵育1小时,测定荧光偏振值(FP),通过FP变化评估Itraconazole与OSBP的结合能力并计算IC50 [3] |
| 细胞实验 |
1. Hh依赖性癌细胞增殖实验:(1)细胞接种:DAOY或MB031细胞以3×10³个/孔接种于96孔板,过夜培养。(2)药物处理:加入含不同浓度Itraconazole(0、25、50、100、200、400 nM)的培养基,37℃、5% CO₂孵育72小时。(3)活力检测:每孔加10 μL CCK-8试剂,继续孵育2小时,测定450 nm吸光度,计算细胞存活率及IC50 [1]
2. HUVEC管形成实验:(1)Matrigel包被:96孔板每孔加50 μL Matrigel,37℃孵育30分钟形成凝胶。(2)细胞制备与处理:HUVEC消化后,用含不同浓度Itraconazole(0、2、5、10、20 μM)的培养基重悬,以1×10⁴个/孔接种于Matrigel上。(3)观察与定量:孵育6小时后,显微镜下拍摄管网络,用ImageJ软件测量总管长并计算抑制率 [2] 3. 药物联合增殖实验:(1)细胞接种:A549细胞以2×10³个/孔接种于96孔板,培养24小时。(2)联合处理:加入含Itraconazole(0、1、5、10 μM)和紫杉醇(0、5、10、20 nM)的培养基,孵育72小时。(3)活力检测:每孔加10 μL MTT试剂,孵育4小时后弃上清,加150 μL DMSO溶解甲瓒结晶,测定570 nm吸光度,计算联合指数(CI)评估协同效应 [3] |
| 动物实验 |
Animal/Disease Models: Female outbred athymic nude mice (6-7weeks old) injected with Ptch+/− cells[1]
Doses: 75 mg/kg, 100 mg/kg Route of Administration: po (oral gavage); twice per day; for 18 days Experimental Results: Suppressed Hh pathway activity and the growth of medulloblastoma in a mouse allograft model. 1. DAOY medulloblastoma xenograft model: (1) Experimental animals: Male BALB/c nude mice (4–6 weeks old, 18–22 g), acclimated for 1 week. (2) Tumor inoculation: DAOY cells (5×10⁶ cells in 0.2 mL of PBS mixed with Matrigel at 1:1) were subcutaneously injected into the right flank of each mouse. (3) Grouping and administration: When tumors reached ~100 mm³, mice were divided into 2 groups (n=6/group): Control group (oral gavage of 0.2 mL 0.5% CMC-Na twice daily); Itraconazole group (oral gavage of 50 mg/kg Itraconazole dissolved in 0.5% CMC-Na twice daily). (4) Monitoring and sampling: Tumor volume (Volume = length × width² / 2) and body weight were measured every 3 days. After 21 days, mice were euthanized, tumors were excised, weighed, and stored at -80°C for Western blot analysis [1] 2. CAM assay and HUVEC xenograft model: (1) CAM assay: Fertilized chicken eggs were incubated at 37°C with 60% humidity for 3 days. A 1 cm² window was opened on the eggshell, and 10 μL of Itraconazole solution (10 mg/mL in DMSO) or DMSO (control) was added to the CAM. The window was sealed, and incubation continued for 48 hours. Eggs were opened, CAM was photographed, and vascular density was quantified using ImageJ. (2) HUVEC xenograft model: Nude mice were subcutaneously injected with HUVECs (1×10⁷ cells in 0.2 mL PBS). When tumors reached ~80 mm³, mice were divided into 2 groups (n=6/group): Control group (intraperitoneal injection of 0.2 mL normal saline + 5% DMSO daily); Itraconazole group (intraperitoneal injection of 20 mg/kg Itraconazole dissolved in normal saline + 5% DMSO daily). After 14 days, mice were euthanized, and tumors were excised and weighed [2] 3. A549 and PANC-1 xenograft models: (1) A549 model: Nude mice were subcutaneously injected with A549 cells (2×10⁶ cells in 0.2 mL PBS). When tumors reached ~120 mm³, mice were divided into 4 groups (n=6/group): Control (oral gavage of 0.2 mL 0.5% CMC-Na daily); Paclitaxel alone (10 mg/kg paclitaxel in 0.2 mL normal saline, intraperitoneal injection once weekly); Itraconazole alone (100 mg/kg Itraconazole in 0.2 mL 0.5% CMC-Na, oral gavage daily); Combination group (paclitaxel + Itraconazole as above). (2) PANC-1 model: Nude mice were inoculated with PANC-1 cells (3×10⁶ cells in 0.2 mL PBS). When tumors reached ~100 mm³, mice were divided into 2 groups: Control (oral gavage of 0.5% CMC-Na) and Itraconazole (75 mg/kg Itraconazole oral gavage daily). For both models, tumor volume and body weight were measured every 3 days; after 30 days (A549) or 28 days (PANC-1), mice were euthanized, and tumors were collected [3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Itraconazole is rapidly absorbed after oral administration. As oral capsules, peak plasma concentrations of itraconazole are reached within two to five hours. The observed absolute oral bioavailability of itraconazole is about 55%. Itraconazole exposure is lower with the capsule formulation than with the oral solution when the same dose of the drug is given. Maximal drug absorption is achieved under adequate gastric acidity. As a consequence of non-linear pharmacokinetics, itraconazole accumulates in plasma during multiple dosing. Steady-state concentrations are generally reached within about 15 days, with Cmax values of 0.5 μg/mL, 1.1 μg/mL and 2.0 μg/mL after oral administration of 100 mg once daily, 200 mg once daily and 200 mg b.i.d., respectively. Itraconazole is excreted mainly as inactive metabolites in urine (35%) and in feces (54%) within one week of an oral solution dose. Renal excretion of itraconazole and the active metabolite hydroxyitraconazole account for less than 1% of an intravenous dose. Based on an oral radiolabeled dose, fecal excretion of unchanged drug ranges from 3% to 18% of the dose. As the re-distribution of itraconazole from keratinous tissues appears to be negligible, the elimination of itraconazole from these tissues is related to epidermal regeneration. Contrary to plasma, the concentration in skin persists for two to four weeks after discontinuation of a 4-week treatment and in nail keratin – where itraconazole can be detected as early as one week after the start of treatment – for at least six months after the end of a 3-month treatment period. The volume of distribution is more than 700 L in adults. Itraconazole is lipophilic and extensively distributed into tissues. Concentrations in the lung, kidney, liver, bone, stomach, spleen and muscle were found to be two to three times higher than corresponding concentrations in plasma, and the uptake into keratinous tissues, skin in particular, up to four times higher. Concentrations in the cerebrospinal fluid are much lower than in plasma. The mean total plasma clearance following intravenous administration is 278 mL/min. Itraconazole clearance decreases at higher doses due to saturable hepatic metabolism. The pharmacokinetics of itraconazole after intravenous administration and its absolute oral bioavailability from an oral solution were studied in a randomized crossover study in 6 healthy male volunteers. The observed absolute oral bioavailability of itraconazole was 55%. The oral bioavailability of itraconazole is maximal when itraconazole capsules are taken with a full meal. The pharmacokinetics of itraconazole were studied in 6 healthy male volunteers who received, in a crossover design, single 100 mg doses of itraconazole as a polyethylene glycol capsule, with or without a full meal. The same 6 volunteers also received 50 mg or 200 mg with a full meal in a crossover design. In this study, only itraconazole plasma concentrations were measured. The respective pharmacokinetic parameters for itraconazole are presented in the table /provided/. Table: Oral Bioavailability of Itraconazole (Itraconazole capsules): [Table#7579] Metabolism / Metabolites Itraconazole is extensively metabolized in the liver. In vitro studies have shown that CYP3A4 is the major enzyme involved in the metabolism of itraconazole. While itraconazole can be metabolized to more than 30 metabolites, the main metabolite is hydroxyitraconazole. Hydroxyitraconazole has in vitro antifungal activity comparable to itraconazole; trough plasma concentrations of this metabolite are about twice those of the parent compound. Other metabolites include keto-itraconazole and N-dealkyl-itraconazole. Itraconazole is metabolized predominantly by the cytochrome P450 3A4 isoenzyme system (CYP3A4), resulting in the formation of several metabolites, including hydroxyitraconazole, the major metabolite. Results of a pharmacokinetics study suggest that itraconazole may undergo saturable metabolism with multiple dosing. Itraconazole (ITZ) is metabolized in vitro to three inhibitory metabolites: hydroxy-itraconazole (OH-ITZ), keto-itraconazole (keto-ITZ), and N-desalkyl-itraconazole (ND-ITZ). The goal of this study was to determine the contribution of these metabolites to drug-drug interactions caused by ITZ. Six healthy volunteers received 100 mg ITZ orally for 7 days, and pharmacokinetic analysis was conducted at days 1 and 7 of the study. The extent of CYP3A4 inhibition by ITZ and its metabolites was predicted using this data. ITZ, OH-ITZ, keto-ITZ, and ND-ITZ were detected in plasma samples of all volunteers. A 3.9-fold decrease in the hepatic intrinsic clearance of a CYP3A4 substrate was predicted using the average unbound steady-state concentrations (C(ss,ave,u)) and liver microsomal inhibition constants for ITZ, OH-ITZ, keto-ITZ, and ND-ITZ. Accounting for circulating metabolites of ITZ significantly improved the in vitro to in vivo extrapolation of CYP3A4 inhibition compared to a consideration of ITZ exposure alone. Itraconazole is extensively metabolized by the liver into a large number of metabolites, including hydroxyitraconazole, the major metabolite. The main metabolic pathways are oxidative scission of the dioxolane ring, aliphatic oxidation at the 1-methylpropyl substituent, N-dealkylation of this 1-methylpropyl substituent, oxidative degradation of the piperazine ring and triazolone scission. Route of Elimination: Itraconazole is metabolized predominately by the cytochrome P450 3A4 isoenzyme system (CYP3A4) in the liver, resulting in the formation of several metabolites, including hydroxyitraconazole, the major metabolite. Fecal excretion of the parent drug varies between 3-18% of the dose. Renal excretion of the parent drug is less than 0.03% of the dose. About 40% of the dose is excreted as inactive metabolites in the urine. No single excreted metabolite represents more than 5% of a dose. Half Life: 21 hours Biological Half-Life The terminal half-life of itraconazole generally ranges from 16 to 28 hours after a single dose and increases to 34 to 42 hours with repeated dosing. The metabolite of itraconazole is excreted from the plasma more rapidly than the parent compound. Oral bioavailability: In mice, after oral administration of 100 mg/kg Itraconazole, the oral bioavailability was ~40% (calculated by comparing the area under the plasma concentration-time curve (AUC) of oral and intravenous administration) [3] - Plasma half-life (t₁/₂): In mice, the plasma t₁/₂ of Itraconazole was ~8 hours (measured by high-performance liquid chromatography (HPLC) after oral administration of 100 mg/kg) [3] - Tissue distribution: In the A549 lung cancer xenograft model, the concentration of Itraconazole in tumor tissues was ~3 times higher than that in plasma (detected 24 hours after oral administration of 100 mg/kg) [3] - Metabolism: Itraconazole is mainly metabolized by hepatic cytochrome P450 3A4 (CYP3A4) to form inactive hydroxy metabolites, which are excreted in feces and urine [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Itraconazole interacts with 14-α demethylase, a cytochrome P-450 enzyme necessary to convert lanosterol to ergosterol. As ergosterol is an essential component of the fungal cell membrane, inhibition of its synthesis results in increased cellular permeability causing leakage of cellular contents. Itraconazole may also inhibit endogenous respiration, interact with membrane phospholipids, inhibit the transformation of yeasts to mycelial forms, inhibit purine uptake, and impair triglyceride and/or phospholipid biosynthesis. Hepatotoxicity Transient, mild-to-moderate elevations in serum aminotransferase levels occur in 1% to 5% of patients on itraconazole. These elevations are largely asymptomatic and self-limited, resolving even with continuation of therapy. Clinically apparent hepatotoxicity is rare but has been well described and can be severe and even fatal. The liver injury from itraconazole typically presents 1 to 6 months after starting therapy with symptoms of fatigue and jaundice. The pattern of serum enzyme elevations is typically cholestatic (Case 1), but cases of severe hepatitis with acute liver failure typically have a hepatocellular enzyme pattern (Case 2). Immunoallergic features (rash, fever, eosinophilia) are uncommon as is autoantibody formation. Recovery upon stopping therapy can be delayed for several weeks and generally takes 4 to 10 weeks, although in some cases recovery may be prolonged. Likelihood score: B (likely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of itraconazole during breastfeeding. However, limited data indicate that maternal itraconazole produces levels in milk that are less than the 5 mg/kg daily doses that have been recommended to treat infants. Until more data become available, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. If itraconazole is used during breastfeeding, monitoring of the infant’s liver enzymes should be considered, especially with long courses of therapy. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Most of the itraconazole in plasma is bound to protein (99.8%), with albumin being the main binding component (99.6% for the hydroxy-metabolite). It also has a marked affinity for lipids. Only 0.2% of the itraconazole in plasma is present as the free drug. Toxicity Data No significant lethality was observed when itraconazole was administered orally to mice and rats at dosage levels of 320 mg/kg or to dogs at 200 mg/kg. Interactions The class IA antiarrhythmic quinidine and class III antiarrhythmic dofetilide are known to prolong the QT interval. Co-administration of quinidine or dofetilide with itraconazole may increase plasma concentrations of quinidine or dofetilide which could result in serious cardiovascular events. Therefore, concomitant administration of itraconazole and quinidine or dofetilide is contraindicated. The class IA antiarrhythmic disopyramide has the potential to increase the QT interval at high plasma concentrations. Caution is advised when itraconazole and disopyramide are administered concomitantly. Concomitant administration of digoxin and itraconazole has led to increased plasma concentrations of digoxin. Reduced plasma concentrations of itraconazole were reported when itraconazole was administered concomitantly with phenytoin. Carbamazepine, phenobarbital and phenytoin are all inducers of CYP3A4. Although interactions with carbamazepine and phenobarbital have not been studied, concomitant administration of itraconazole and these drugs would be expected to result in decreased plasma concentrations of itraconazole. Drug interaction studies have demonstrated that plasma concentrations of azole antifungal agents and their metabolites, including itraconazole and hydroxyitraconazole, were significantly decreased when these agents were given concomitantly with rifabutin or rifampin. In vivo data suggest that rifabutin is metabolized in part by CYP3A4. Itraconazole may inhibit the metabolism of rifabutin. Itraconazole may inhibit the metabolism of busulfan, docetaxel and vinca alkaloids. For more Interactions (Complete) data for Itraconazole (29 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral >320 mg/kg LD50 Mouse oral >320 mg/kg LD50 Dog oral >200 mg/kg LD50 Guinea pig oral >160 mg/kg 1. In vivo toxicity in mice: After 21 days of oral administration of 50 mg/kg Itraconazole twice daily, no significant body weight loss was observed in nude mice (body weight change: ~+5% vs. ~+6% in control). Serum levels of alanine transaminase (ALT) and aspartate transaminase (AST) were not significantly different from the control group (P>0.05) [1] 2. Renal toxicity assessment: After 14 days of intraperitoneal injection of 20 mg/kg Itraconazole daily, serum creatinine (Cr) and blood urea nitrogen (BUN) levels in nude mice were within the normal range, with no significant difference from the control group (P>0.05) [2] 3. Plasma protein binding and hematotoxicity: In vitro human plasma binding assay showed that the plasma protein binding rate of Itraconazole was ~99%. After 30 days of combined administration of 100 mg/kg Itraconazole (oral) and 10 mg/kg paclitaxel (intraperitoneal), the peripheral blood white blood cell count of nude mice was normal (~6×10⁹/L), indicating no significant myelosuppression. Serum bilirubin levels were normal, suggesting no hepatotoxicity [3] |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Antifungal Agents; Antiprotozoal Agents Itraconazole capsules are indicated for the treatment of the following fungal infections in immunocompromised and non-immunocompromised patients: Blastomycosis, pulmonary and extrapulmonary; Histoplasmosis, including chronic cavitary pulmonary disease and disseminated, non-meningeal histoplasmosis and Aspergillosis, pulmonary and extrapulmonary, in patients who are intolerant of or who are refractory to amphotericin B therapy. /Included in US product label/ Itraconazole capsules are also indicated for the treatment of the following fungal infections in non-immunocompromised patients: Onychomycosis of the toenail, with or without fingernail involvement, due to dermatophytes (tinea unguium) and Onychomycosis of the fingernail due to dermatophytes (tinea unguium). /Included in US product label/ Drug Warnings /BOXED WARNING/ Congestive Heart Failure, Cardiac Effects: Itraconazole capsules should not be administered for the treatment of onychomycosis in patients with evidence of ventricular dysfunction such as congestive heart failure (CHF) or a history of CHF. If signs or symptoms of congestive heart failure occur during administration of itraconazole capsules, discontinue administration. When itraconazole was administered intravenously to dogs and healthy human volunteers, negative inotropic effects were seen. /BOXED WARNING/ Drug Interactions: Coadministration of the following drugs are contraindicated with itraconazole capsules: methadone, disopyramide, dofetilide, dronedarone, quinidine, ergot alkaloids (such as dihydroergotamine, ergometrine (ergonovine), ergotamine, methylergometrine (methylergonovine)), irinotecan, lurasidone, oral midazolam, pimozide, triazolam, felodipine, nisoldipine, ranolazine, eplerenone, cisapride, lovastatin, simvastatin and, in subjects with renal or hepatic impairment, colchicine. Coadministration with itraconazole can cause elevated plasma concentrations of these drugs and may increase or prolong both the pharmacologic effects and/or adverse reactions to these drugs. For example, increased plasma concentrations of some of these drugs can lead to QT prolongation and ventricular tachyarrhythmias including occurrences of torsades de pointes, a potentially fatal arrhythmia. Itraconazole is contraindicated in patients with known hypersensitivity to the drug or any ingredient in the formulation. Although information concerning cross-sensitivity between itraconazole and other triazole or imidazole antifungal agents is not available, the manufacturer states that itraconazole should be used with caution in individuals hypersensitive to other azoles. Adverse GI effects have been reported in about 1-11% of patients receiving IV or oral itraconazole for the treatment of systemic fungal infections or oropharyngeal or esophageal candidiasis or for empiric anti-fungal therapy. These adverse GI effects usually are transient and respond to symptomatic treatment without alteration of itraconazole therapy; however, reduction of dosage or discontinuance of the drug occasionally may be required. For more Drug Warnings (Complete) data for Itraconazole (27 total), please visit the HSDB record page. Pharmacodynamics Itraconazole is an antifungal agent that inhibits cell growth and promotes cell death of fungi. It exhibits in vitro activity against _Blastomyces dermatitidis_, _Histoplasma capsulatum_, _Histoplasma duboisii_, _Aspergillus flavus_, _Aspergillus fumigatus_, and _Trichophyton_ species. 1. Clinical background and new indications: Itraconazole is an FDA-approved triazole antifungal agent originally indicated for the treatment of superficial and systemic fungal infections (e.g., aspergillosis, candidiasis). This study identified its new pharmacological activity: inhibiting the Hedgehog (Hh) signaling pathway, making it a potential therapeutic agent for Hh-dependent cancers (e.g., medulloblastoma, basal cell carcinoma) [1] 2. Anti-angiogenic mechanism: The anti-angiogenic effect of Itraconazole is mediated by dual inhibition of VEGFR2 (blocking VEGF-induced endothelial cell activation) and PI3K (suppressing downstream survival signals in endothelial cells). This dual mechanism suggests its potential application in angiogenesis-related diseases, such as age-related macular degeneration and metastatic cancer [2] 3. Drug repurposing value: Itraconazole has clinical safety data (approved for fungal infections), making it suitable for repurposing as an anticancer agent. When combined with chemotherapeutic drugs (e.g., paclitaxel), it enhances antitumor efficacy and reduces the required dose of chemotherapeutics, thereby minimizing chemotherapy-related toxicity. A phase I clinical trial of Itraconazole combined with paclitaxel for advanced solid tumors was initiated in 2015 [3] |
| 分子式 |
C35H38CL2N8O4
|
|---|---|
| 分子量 |
705.65
|
| 精确质量 |
704.239
|
| CAS号 |
84625-61-6
|
| 相关CAS号 |
Hydroxy Itraconazole;112559-91-8;Hydroxy Itraconazole-d8;Itraconazole-d5;1217510-38-7;Itraconazole-d3;1217512-35-0;Itraconazole-d9;1309272-50-1
|
| PubChem CID |
55283
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
850.0±75.0 °C at 760 mmHg
|
| 熔点 |
166°C
|
| 闪点 |
467.9±37.1 °C
|
| 蒸汽压 |
0.0±3.2 mmHg at 25°C
|
| 折射率 |
1.678
|
| LogP |
4.35
|
| tPSA |
104.7
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
11
|
| 重原子数目 |
49
|
| 分子复杂度/Complexity |
1120
|
| 定义原子立体中心数目 |
2
|
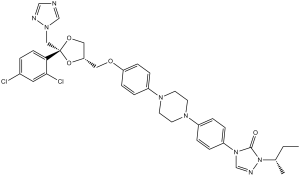
| SMILES |
CCC(C)N1C(=O)N(C=N1)C2=CC=C(C=C2)N3CCN(CC3)C4=CC=C(C=C4)OC[C@H]5CO[C@](O5)(CN6C=NC=N6)C7=C(C=C(C=C7)Cl)Cl
|
| InChi Key |
VHVPQPYKVGDNFY-ZPGVKDDISA-N
|
| InChi Code |
InChI=1S/C35H38Cl2N8O4/c1-3-25(2)45-34(46)44(24-40-45)29-7-5-27(6-8-29)41-14-16-42(17-15-41)28-9-11-30(12-10-28)47-19-31-20-48-35(49-31,21-43-23-38-22-39-43)32-13-4-26(36)18-33(32)37/h4-13,18,22-25,31H,3,14-17,19-21H2,1-2H3/t25?,31-,35-/m0/s1
|
| 化学名 |
2-butan-2-yl-4-[4-[4-[4-[[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-1,2,4-triazol-3-one
|
| 别名 |
R51211, Orungal, Oriconazole, Sporanox, R 51211; R-51211, Itraconazole, Itraconazolum, Itraconazol, Itrizole
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 0.62 mg/mL (0.88 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 6.2 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 0.62 mg/mL (0.88 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 6.2 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 View More
配方 3 中的溶解度: 5% DMSO+70% PEG 300+ddH2O: 9mg/mL 配方 4 中的溶解度: 20 mg/mL (28.34 mM) in 0.5% CMC-Na/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4171 mL | 7.0857 mL | 14.1713 mL | |
| 5 mM | 0.2834 mL | 1.4171 mL | 2.8343 mL | |
| 10 mM | 0.1417 mL | 0.7086 mL | 1.4171 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05563766 | Not yet recruiting | Drug: Itraconazole | Esophageal Adenocarcinoma Esophageal Squamous Cell Carcinoma |
VA Office of Research and Development | May 1, 2024 | Phase 2 |
| NCT05609253 | Recruiting | Drug: Itraconazole in capsule form Drug: Itraconazole in solution form |
Barrett Oesophagitis With Dysplasia | University of Kansas Medical Center | September 14, 2022 | Phase 1 |
| NCT04018872 | Recruiting | Drug: Itraconazole | Esophagus Adenocarcinoma Esophagus Squamous Cell Carcinoma |
Dallas VA Medical Center | June 24, 2019 | Phase 2 |
| NCT03572049 | Completed Has Results | Drug: SUBA itraconazole Drug: Conventional itraconazole |
Invasive Fungal Infections | University of Alabama at Birmingham | September 17, 2018 | Phase 2 Phase 3 |