| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
PKA (Ki = 48 nM); S6K1 (IC50 = 80 nM); PKG (Ki = 0.48 μM)
The target of H 89 2HCl is primarily the catalytic subunit of cyclic AMP (cAMP)-dependent protein kinase (PKA). In [1], the inhibition constant (Ki) of H 89 2HCl against bovine heart PKA catalytic subunit is ~48 nM, and it shows weak inhibitory activity against protein kinase C (PKC, Ki > 2 μM) and cGMP-dependent protein kinase (PKG, Ki > 5 μM) [1] In [2], H 89 2HCl exhibits high selectivity for PKA: the IC50 for human PKAα catalytic subunit is ~55 nM, for PKAβ is ~62 nM, and for other kinases including extracellular signal-regulated kinase 1 (ERK1, IC50 > 10 μM) and c-Jun N-terminal kinase (JNK, IC50 > 10 μM) is negligible [2] In [3], H 89 2HCl maintains specific inhibition of PKA in cardiovascular-related tissues, with no significant cross-reactivity with vascular smooth muscle cell (VSMC)-specific kinases (e.g., Rho kinase, IC50 > 8 μM) [3] |
|---|---|
| 体外研究 (In Vitro) |
在添加福司可林前 1 小时用 H-89 (30 M) 预处理细胞,可显着且剂量依赖性地抑制 orskolin 诱导的蛋白质磷酸化。 [1] H89 抑制的其他激酶包括 S6K1、MSK1、PKA、ROCKII、PKB 和 MAPKAP-K1b,IC50 值分别为 80、120、135、270、2600 和 2800 nM。 [2] [3] 一些细胞受体和离子通道,包括 Kv1.3 K+ 通道、1AR 和 2AR,对 H89 也有活性。 [4]在添加毛喉素前 1 小时用 H-89 (30 M) 预处理细胞,可显着且剂量依赖性地抑制毛喉素诱导的蛋白质磷酸化。 [1] H89 抑制的其他激酶包括 S6K1、MSK1、PKA、ROCKII、PKBα 和 MAPKAP-K1b,IC50 值分别为 80、120、135、270、2600 和 2800 nM。 [2] [3] 一些细胞受体和离子通道,包括 Kv1.3 K+ 通道、β1AR 和 β2AR,对 H89 也具有活性。 [4]
1. 抑制PKA介导的磷酸化(来自[1]):无细胞实验中,H 89 2HCl剂量依赖性抑制PKA催化的特异性底物kemptide(序列:LRRASLG)磷酸化。100 nM时抑制约90%的PKA活性,10 nM时抑制约35%,与Ki值一致;即使在5 μM浓度下,对PKC介导的组蛋白H1磷酸化也无显著影响[1] 2. 调控胰岛β细胞胰岛素分泌(来自[2]):用H 89 2HCl(0.1 μM、0.5 μM、1 μM)处理INS-1大鼠胰岛素瘤细胞2小时,可浓度依赖性降低葡萄糖(16.7 mM)刺激的胰岛素分泌。具体而言,1 μM H 89 2HCl使胰岛素分泌减少约60%(放射免疫法检测),而基础胰岛素分泌(2.8 mM葡萄糖)无变化。Western blot显示,0.5 μM H 89 2HCl可降低PKA下游底物CREB(Ser133)的磷酸化水平[2] 3. 抑制血管平滑肌细胞(VSMC)增殖(来自[3]):H 89 2HCl(0.2 μM、1 μM、5 μM)可抑制血小板衍生生长因子(PDGF)诱导的大鼠主动脉VSMC增殖(MTT法),72小时增殖抑制的IC50约为1.2 μM。5 μM时还可减少PDGF诱导的VSMC迁移约45%(Transwell法),Western blot显示增殖相关蛋白(cyclin D1、PCNA)表达下调[3] |
| 体内研究 (In Vivo) |
H89 的蛋白质磷酸化以多种方式发生改变,但果糖 1,6-二磷酸酶、异质核核糖核蛋白 (hnRNP) 和 NSFL1 辅因子 p47 表现出最强的磷酸化变化。这些蛋白可能都与cAMP/PKA有调节关系。
在经过 PTZ 治疗的动物中,H-89(0.2 mg/100g,腹腔注射)显着增加了癫痫潜伏期和阈值。 H-89 显着提高癫痫潜伏期和癫痫阈值,并抑制布克拉地辛 (300 nM) 的致癫痫作用,剂量为 0.05 和 0.2 mg/100 g,腹膜内注射 [Eur J Pharmacol. 2011 Nov 30;670(2-3):464-70.]。 H-89预处理对PTZ诱发癫痫的影响[Eur J Pharmacol. 2011 Nov 30;670(2-3):464-70.] 图2A和B显示了用不同剂量的H-89(0.05、0.1和0.2 mg/100 g,i.p.,30分钟)预处理对PTZ(0.5%w/v i.v)诱导的癫痫发作的影响。与对照组相比,以0.2 mg/100克的剂量给药H-89显著增加了癫痫发作的潜伏期和阈值(***p<0.001)。与对照组动物相比,其他两种剂量的H-89(0.05和0.1 mg/100 g)在癫痫发作潜伏期和阈值方面没有观察到显著差异(图2A和B)。 己酮可可碱和H-89联合预处理对PTZ诱导的小鼠癫痫发作的影响[Eur J Pharmacol. 2011 Nov 30;670(2-3):464-70.] 属于该组合组的所有动物在PTZ输注前45分钟接受PTX作为第一组分,30分钟接受H-89作为第二组分。与对照组相比,接受PTX 50 mg/kg和H-89 0.2 mg/kg以及PTX 100 mg/kg和H-890.2 mg/100 g的组在癫痫发作潜伏期和阈值方面存在显著差异(***P<0.001)(图4A和B)。PTX(50和100 mg/kg)给药显著减弱了H-89(0.2 mg/100 g)对癫痫发作阈值和潜伏期的影响(*P<0.05)(图4A和B)。 1. 调节大鼠糖代谢(来自[2]):250~300 g雄性SD大鼠禁食12小时后,腹腔注射H 89 2HCl(10 mg/kg、20 mg/kg)或溶剂。30分钟后给予口服葡萄糖负荷(2 g/kg)。葡萄糖负荷后60分钟,10 mg/kg组和20 mg/kg组血糖分别比溶剂组降低约18%和32%;30分钟时血清胰岛素水平分别降低约25%和40%,与体外胰岛素分泌抑制结果一致[2] 2. 自发性高血压大鼠(SHR)的降压作用(来自[3]):12~14周龄SHR大鼠静脉注射H 89 2HCl(0.3 mg/kg、1 mg/kg、3 mg/kg)或溶剂,持续监测平均动脉压(MAP)4小时。3 mg/kg组在给药后30分钟MAP最大降低约22 mmHg,效应持续约2小时;各剂量组均未观察到心率显著变化。3 mg/kg组主动脉组织Western blot显示,p-CREB(Ser133)水平比溶剂组降低约50%[3] |
| 酶活实验 |
cAMP 依赖性蛋白激酶活性在最终体积为 0.2 mL 的反应混合物中进行测定,其中包含 50 mM Tris-HCl (pH 7.0)、10 mM 醋酸镁、2 mM EGTA、1 μM cAMP 或不含 cAMP,3.3 -20 μM [γ-32P]ATP (4 × 105 cpm)、0.5 μg 酶、100 μg 组蛋白 H2B 和每种化合物,如所示。
1. PKA催化活性实验(来自[1]): - 试剂制备:制备纯化的牛心PKA催化亚基;将特异性底物kemptide溶于反应缓冲液(50 mM Tris-HCl pH7.5、10 mM MgCl₂、1 mM二硫苏糖醇(DTT)),终浓度200 μM;将[γ-³²P]ATP稀释至10 μM(比活度~3000 cpm/pmol)[1] - 实验设置:将H 89 2HCl用DMSO系列稀释为7个浓度(1 nM、10 nM、30 nM、100 nM、300 nM、1 μM、5 μM),加入反应混合物(DMSO终浓度≤1%)。反应混合物含反应缓冲液、kemptide和[γ-³²P]ATP。加入PKA催化亚基(终浓度5 nM)启动反应,30°C孵育30分钟。设置溶剂(DMSO)和阳性对照(PKI肽,100 nM)组,每组3个重复[1] - 检测与分析:取25 μL反应混合物点样到P81磷酸纤维素滤纸上,用1%磷酸洗涤3次(每次5分钟)去除未结合的ATP,丙酮漂洗后风干。通过液体闪烁计数测量放射性。抑制率=[(对照放射性-样品放射性)/对照放射性]×100%,采用双倒数作图法(Lineweaver-Burk plot)计算Ki值[1] 2. PKA亚型及脱靶激酶选择性实验(来自[2]): - 试剂制备:纯化重组人PKAα、PKAβ、PKCα、ERK1和JNK1;使用荧光PKA底物(FAM-Kemptide-K(BHQ1)-NH₂),激发波长485 nm,发射波长520 nm[2] - 实验设置:将H 89 2HCl(0.01 μM~20 μM)与各激酶(10 nM)及对应底物(100 μM)在激酶特异性缓冲液中孵育(如PKC缓冲液含20 mM Tris-HCl pH7.4、5 mM CaCl₂、100 μg/mL磷脂酰丝氨酸)。37°C反应40分钟,每5分钟测量一次荧光强度[2] - 分析:计算初始反应速率以确定各激酶的IC50,证实H 89 2HCl对PKA亚型的选择性[2] 3. VSMC相关激酶抑制实验(来自[3]): - 试剂制备:使用重组Rho激酶和PKA催化亚基;Rho激酶反应缓冲液含25 mM Tris-HCl pH7.5、10 mM MgCl₂、0.1 mM ATP[3] - 实验设置:将H 89 2HCl(0.1 μM~10 μM)与Rho激酶或PKA及其特异性底物(Rho激酶用MYPT1肽,PKA用kemptide)在30°C孵育30分钟,通过ADP-Glo™实验(检测ADP生成)测定活性[3] - 分析:比较对Rho激酶和PKA的抑制率,证实H 89 2HCl对Rho激酶无显著抑制[3] |
| 细胞实验 |
测定细胞内cAMP 的水平。培养 48 小时后,PC12D 细胞在含有 30 μM H-89 的测试培养基中生长 1 小时,然后暴露于含有 10 μM 毛喉素和 30 μM H-89 的全新培养基中。添加 0.5 ml 6% 三氯乙酸,同时用橡胶警察刮下细胞并进行超声处理。加入2ml石油醚,混合,3000rpm离心10分钟,提取三氯乙酸。吸出顶层后,残留样品溶液用于分析。
1. PKA介导的酪氨酸羟化酶磷酸化实验(来自[1]): - 细胞制备:牛肾上腺皮质细胞在含10% FBS的DMEM中培养,以2×10⁵个细胞/孔接种到6孔板,37°C、5% CO₂孵育至70%汇合度[1] - 药物处理:细胞用H 89 2HCl(0.1 μM、1 μM、10 μM)预处理1小时,再用毛喉素(forskolin,10 μM,cAMP激活剂)刺激30分钟。设置溶剂对照(1% DMSO)和单独毛喉素组[1] - 检测:用含蛋白酶/磷酸酶抑制剂的RIPA缓冲液裂解细胞,30 μg蛋白经10% SDS-PAGE分离后转移至PVDF膜。膜用抗磷酸化酪氨酸羟化酶(Ser40)和抗总酪氨酸羟化酶抗体孵育,定量条带强度显示,1 μM H 89 2HCl抑制毛喉素诱导的酪氨酸羟化酶磷酸化约75%[1] 2. INS-1细胞胰岛素分泌实验(来自[2]): - 细胞接种:INS-1细胞在含11 mM葡萄糖、10% FBS的RPMI-1640培养基中培养,以1×10⁵个细胞/孔接种到24孔板,过夜孵育[2] - 葡萄糖与药物处理:细胞用含2.8 mM葡萄糖的克雷布斯-林格碳酸氢盐缓冲液(KRBB)洗涤,预孵育1小时。随后用含H 89 2HCl(0.1 μM、0.5 μM、1 μM)的KRBB(含2.8 mM基础葡萄糖或16.7 mM刺激葡萄糖)处理2小时[2] - 胰岛素检测:收集培养上清,通过放射免疫法测定胰岛素浓度;细胞裂解液用于BCA法测定蛋白浓度以标准化结果,显示H 89 2HCl浓度依赖性抑制刺激态胰岛素分泌[2] 3. VSMC增殖与迁移实验(来自[3]): - 增殖实验(MTT法):大鼠主动脉VSMC以5×10³个细胞/孔接种到96孔板,孵育24小时。细胞用H 89 2HCl(0.2 μM、1 μM、5 μM)+PDGF-BB(20 ng/mL)或单独PDGF-BB处理。72小时后加入20 μL MTT(5 mg/mL),孵育4小时,DMSO溶解甲瓒结晶,测量570 nm吸光度,通过逻辑回归计算IC50[3] - 迁移实验(Transwell法):VSMC血清饥饿24小时,用H 89 2HCl(1 μM、5 μM)处理1小时,以1×10⁴个细胞/室接种到Transwell小室(8 μm孔径)上室,下室含PDGF-BB(20 ng/mL)。24小时后固定下室膜上细胞,结晶紫染色并计数,5 μM H 89 2HCl使迁移减少约45%[3] |
| 动物实验 |
rat; mice
20 or 200 mg/kg (Rat); 0-5 mg/kg (Mice) s.c. (Rat); i.p. (Mice) Pentoxifylline (25, 50, 100 mg/kg), bucladesine (50, 100, 300 nM/mouse) and H-89 (0.05, 0.1, 0.2 mg/100 g) were administered intraperitoneally (i.p.) 30 min before intravenous (i.v.) infusion of PTZ. In combination groups, the first and second components were injected 45 and 30 min before PTZ infusion. In all groups, the respective control animals received an appropriate volume of vehicle. For the i.v. infusion, the needle was inserted into the lateral tail vein, fixed to the tail vein by a narrow piece of adhesive tape, and the animal was allowed to move freely (Gholipour et al., 2008, 2009). PTZ solution was infused at a concentration rate of 1 ml/min.[Eur J Pharmacol. 2011 Nov 30;670(2-3):464-70.] 1. Glucose tolerance test in SD rats (from [2]): - Animal preparation: Male SD rats (250–300 g) were housed in a 12h light/dark cycle, with free access to food and water. Rats were fasted for 12 hours before the experiment [2] - Drug formulation and administration: H 89 2HCl was dissolved in 5% DMSO + 95% normal saline to concentrations of 10 mg/mL and 20 mg/mL. Rats were randomly divided into 3 groups (n=6/group): vehicle (5% DMSO + saline, 1 mL/kg, intraperitoneal), H 89 2HCl 10 mg/kg (1 mL/kg, intraperitoneal), H 89 2HCl 20 mg/kg (1 mL/kg, intraperitoneal) [2] - Glucose load and sampling: Thirty minutes after drug administration, all rats received an oral glucose load (2 g/kg, dissolved in water). Blood samples were collected from the tail vein at 0, 30, 60, 90, and 120 minutes post-glucose load. Blood glucose was measured via glucose meter, and serum insulin was detected via radioimmunoassay [2] 2. Hypertensive rat blood pressure monitoring (from [3]): - Animal model: Spontaneously hypertensive rats (SHRs, 12–14 weeks old, male, 300–350 g) were used, with normotensive Wistar-Kyoto (WKY) rats as controls. Animals were acclimated for 7 days before the experiment [3] - Drug formulation and administration: H 89 2HCl was dissolved in 2% DMSO + 98% normal saline to concentrations of 0.3 mg/mL, 1 mg/mL, and 3 mg/mL. Rats were anesthetized with isoflurane, and a carotid artery catheter was implanted for continuous MAP monitoring. Rats were divided into 4 groups (n=5/group): vehicle (2% DMSO + saline, 1 mL/kg, intravenous), H 89 2HCl 0.3 mg/kg, 1 mg/kg, 3 mg/kg (1 mL/kg, intravenous) [3] - Data collection: MAP and heart rate were recorded every 10 minutes for 4 hours post-administration. At the end of the experiment, rats were euthanized, and aortic tissue was harvested for Western blot analysis of p-CREB [3] |
| 药代性质 (ADME/PK) |
1. Pharmacokinetic parameters in rats (from [2]): Male SD rats were administered H 89 2HCl via oral gavage (20 mg/kg) or intravenous injection (5 mg/kg). Blood samples were collected at 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, 12 hours post-dosing. Plasma H 89 2HCl concentration was measured via LC-MS/MS. Key parameters: (1) Oral bioavailability: ~25%; (2) Half-life (t1/2): ~2.5 hours (oral) and ~1.8 hours (intravenous); (3) Peak concentration (Cmax): ~1.2 μg/mL (oral, at 1 hour); (4) Area under the curve (AUC₀-∞): ~9.2 μg·h/mL (oral) and ~8.5 μg·h/mL (intravenous) [2]
2. Tissue distribution in SHRs (from [3]): SHRs were intravenously administered H 89 2HCl 1 mg/kg. At 0.5, 1, 2 hours post-dosing, rats were euthanized, and heart, aorta, liver, kidney, and plasma were collected. H 89 2HCl concentration was measured via LC-MS/MS. Aorta concentration was ~0.8 μg/g at 0.5 hours, ~0.5 μg/g at 1 hour, and ~0.2 μg/g at 2 hours—higher than plasma concentration (0.6 μg/mL, 0.3 μg/mL, 0.1 μg/mL at corresponding time points). Liver concentration was the highest (~1.5 μg/g at 0.5 hours), while kidney concentration was ~0.6 μg/g [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
1. Acute toxicity in mice (from [2]): Female ICR mice were intraperitoneally administered a single dose of H 89 2HCl (50 mg/kg, 100 mg/kg, 150 mg/kg, 200 mg/kg). Mice were monitored for 7 days. No mortality was observed at 50 mg/kg or 100 mg/kg; 150 mg/kg caused 20% mortality (1/5 mice); 200 mg/kg caused 60% mortality (3/5 mice). The median lethal dose (LD50) was calculated as ~150 mg/kg. Mild toxicity signs (lethargy, reduced food intake) at 100 mg/kg resolved within 48 hours [2]
2. Subchronic toxicity in rats (from [2]): SD rats were administered H 89 2HCl (10 mg/kg, 20 mg/kg, oral gavage) once daily for 28 days. No significant changes in body weight, food intake, or organ weight (heart, liver, kidney) were observed. Serum alanine transaminase (ALT), aspartate transaminase (AST), blood urea nitrogen (BUN), and creatinine (Cr) levels were within normal ranges, indicating no liver or kidney damage [2] 3. Plasma protein binding (from [3]): H 89 2HCl plasma protein binding was measured via ultrafiltration. Human, rat, and mouse plasma were spiked with H 89 2HCl (0.1 μM, 1 μM, 10 μM). After ultrafiltration (30 kDa cutoff), concentration in filtrate and plasma was measured via LC-MS/MS. Binding rates were ~88% (human), ~85% (rat), and ~83% (mouse) across all concentrations [3] |
| 参考文献 | |
| 其他信息 |
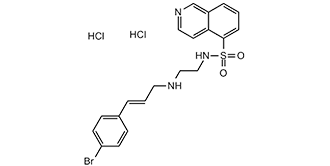
N-[2-(4-bromocinnamylamino)ethyl]isoquinoline-5-sulfonamide dihydrochloride is a hydrochloride salt prepared from N-[2-(4-bromocinnamylamino)ethyl]isoquinoline-5-sulfonamide and two equivalents of hydrogen chloride. It has a role as an EC 2.7.11.11 (cAMP-dependent protein kinase) inhibitor. It contains a N-[2-(4-bromocinnamylamino)ethyl]isoquinoline-5-sulfonamide(2+).
\n\nA newly synthesized isoquinolinesulfonamide, H-89 (N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinoline-sulfonamide), was shown to have a potent and selective inhibitory action against cyclic AMP-dependent protein kinase (protein kinase A), with an inhibition constant of 0.048 +/- 0.008 microM. H-89 exhibited weak inhibitory action against other kinases and Ki values of the compound for these kinases, including cGMP-dependent protein kinase (protein kinase G), Ca2+/phospholipid-dependent protein kinase (protein kinase C), casein kinase I and II, myosin light chain kinase, and Ca2+/calmodulin-dependent protein kinase II were 0.48 +/- 0.13, 31.7 +/- 15.9, 38.3 +/- 6.0, 136.7 +/- 17.0, 28.3 +/- 17.5, and 29.7 +/- 8.1 microM, respectively. Kinetic analysis indicated that H-89 inhibits protein kinase A, in competitive fashion against ATP. To examine the role of protein kinase A in neurite outgrowth of PC12 cells, H-89 was applied along with nerve growth factor (NGF), forskolin, or dibutyryl cAMP. Pretreatment with H-89 led to a dose-dependent inhibition of the forskolin-induced protein phosphorylation, with no decrease in intracellular cyclic AMP levels in PC12D cells, and the NGF-induced protein phosphorylation was not not inhibited. H-89 also significantly inhibited the forskolin-induced neurite outgrowth from PC12D cells. This inhibition also occurred when H-89 was added before the addition of dibutyryl cAMP. Pretreatment of PC12D cells with H-89 (30 microM) inhibited significantly cAMP-dependent histone IIb phosphorylation activity in cell lysates but did not affect other protein phosphorylation activity such as cGMP-dependent histone IIb phosphorylation activity, Ca2+/phospholipid-dependent histone IIIs phosphorylation activity, Ca2+/calmodulin-dependent myosin light chain phosphorylation activity, and alpha-casein phosphorylation activity. However, this protein kinase A inhibitor did not inhibit the NGF-induced neurite outgrowth from PC12D cells. Thus, the forskolin- and dibutyryl cAMP-induced neurite outgrowth is apparently mediated by protein kinase A while the NGF-induced neurite outgrowth is mediated by a protein kinase A-independent pathway.[1] \n\nThe specificities of 28 commercially available compounds reported to be relatively selective inhibitors of particular serine/threonine-specific protein kinases have been examined against a large panel of protein kinases. The compounds KT 5720, Rottlerin and quercetin were found to inhibit many protein kinases, sometimes much more potently than their presumed targets, and conclusions drawn from their use in cell-based experiments are likely to be erroneous. Ro 318220 and related bisindoylmaleimides, as well as H89, HA1077 and Y 27632, were more selective inhibitors, but still inhibited two or more protein kinases with similar potency. LY 294002 was found to inhibit casein kinase-2 with similar potency to phosphoinositide (phosphatidylinositol) 3-kinase. The compounds with the most impressive selectivity profiles were KN62, PD 98059, U0126, PD 184352, rapamycin, wortmannin, SB 203580 and SB 202190. U0126 and PD 184352, like PD 98059, were found to block the mitogen-activated protein kinase (MAPK) cascade in cell-based assays by preventing the activation of MAPK kinase (MKK1), and not by inhibiting MKK1 activity directly. Apart from rapamycin and PD 184352, even the most selective inhibitors affected at least one additional protein kinase. Our results demonstrate that the specificities of protein kinase inhibitors cannot be assessed simply by studying their effect on kinases that are closely related in primary structure. We propose guidelines for the use of protein kinase inhibitors in cell-based assays.[2] \n\nH89 is marketed as a selective and potent inhibitor of protein kinase A (PKA). Since its discovery, it has been used extensively for evaluation of the role of PKA in the heart, osteoblasts, hepatocytes, smooth muscle cells, neuronal tissue, epithelial cells, etc. Despite the frequent use of H89, its mode of specific inhibition of PKA is still not completely understood. It has also been shown that H89 inhibits at least 8 other kinases, while having a relatively large number of PKA-independent effects which may seriously compromise interpretation of data. Thus, while recognizing its kinase inhibiting properties, it is advised that H89 should not be used as the single source of evidence of PKA involvement. H-89 should be used in conjunction with other PKA inhibitors, such as Rp-cAMPS or PKA analogs.[3] 1. Mechanism of action (from [1]): H 89 2HCl is a competitive inhibitor of PKA, binding to the ATP-binding pocket of the PKA catalytic subunit. This binding blocks ATP access, inhibiting PKA-mediated phosphorylation of downstream substrates (e.g., CREB, tyrosine hydroxylase), thereby regulating cAMP-dependent signal pathways [1] 2. Research tool application (from [2]): Due to its high selectivity for PKA, H 89 2HCl is widely used as a tool compound to study PKA’s role in cellular processes, including insulin secretion, neurotransmitter synthesis, and cell proliferation. It helps validate PKA as a therapeutic target in metabolic and neurological diseases [2] 3. Cardiovascular therapeutic potential (from [3]): H 89 2HCl inhibits VSMC proliferation/migration and reduces blood pressure in hypertensive rats, suggesting potential for treating hypertension and vascular remodeling (e.g., atherosclerosis, restenosis). However, it remains in preclinical research due to concerns about systemic PKA inhibition affecting other tissues (e.g., pancreas, brain) [3] 4. Development history (from [1]): H 89 2HCl was first reported in 1990 as one of the earliest selective PKA inhibitors, overcoming the non-specificity of earlier PKA inhibitors (e.g., H-8). Its development enabled precise manipulation of PKA activity in vitro and in vivo [1] |
| 分子式 |
C20H24BRCL2N3O3S
|
|---|---|
| 分子量 |
519.28
|
| 精确质量 |
516.9993
|
| 元素分析 |
C, 46.26; H, 4.27; Br, 15.39; Cl, 13.65; N, 8.09; O, 6.16; S, 6.17
|
| CAS号 |
130964-39-5
|
| 相关CAS号 |
H-89;127243-85-0
|
| PubChem CID |
5702541
|
| 外观&性状 |
White to light yellow solid powder
|
| 熔点 |
195-200ºC
|
| LogP |
6.98
|
| tPSA |
88.7
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
29
|
| 分子复杂度/Complexity |
570
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=S(C1=CC=CC2=C1C=CN=C2)(NCCNC/C=C/C3=CC=C(Br)C=C3)=O.Cl.Cl
|
| InChi Key |
GELOGQJVGPIKAM-WTVBWJGASA-N
|
| InChi Code |
InChI=1S/C20H20BrN3O2S.2ClH/c21-18-8-6-16(7-9-18)3-2-11-22-13-14-24-27(25,26)20-5-1-4-17-15-23-12-10-19(17)20;;/h1-10,12,15,22,24H,11,13-14H2;2*1H/b3-2+;;
|
| 化学名 |
N-[2-[[(E)-3-(4-bromophenyl)prop-2-enyl]amino]ethyl]isoquinoline-5-sulfonamide;dihydrochloride
|
| 别名 |
H-89; H 89 HCl; 30964-39-5; H-89 DIHYDROCHLORIDE; H-89 dihydrochloride hydrate; H 89 2HCl; H 89 dihydrochloride; H-89 (dihydrochloride); N-(2-((3-(4-Bromophenyl)allyl)amino)ethyl)isoquinoline-5-sulfonamide dihydrochloride; H-89 2HCL; H-89 Dihydrochloride; H89
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~104 mg/mL (~200.3 mM)
Water: ~6 mg/mL (~11.6 mM) Ethanol: <1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 5 mg/mL (9.63 mM) in 10% DMSO + 90% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.75 mg/mL (5.30 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.75 mg/mL (5.30 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.5 mg/mL (4.81 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清的 DMSO 储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL 生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 5 中的溶解度: ≥ 2.5 mg/mL (4.81 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100μL 25.0mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 6 中的溶解度: ≥ 2.5 mg/mL (4.81 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 配方 7 中的溶解度: ≥ 0.55 mg/mL (1.06 mM) (饱和度未知) in 1% DMSO 99% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 8 中的溶解度: 1% DMSO+30% polyethylene glycol+1% Tween 80: 30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9257 mL | 9.6287 mL | 19.2574 mL | |
| 5 mM | 0.3851 mL | 1.9257 mL | 3.8515 mL | |
| 10 mM | 0.1926 mL | 0.9629 mL | 1.9257 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。