| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
| 靶点 |
β-catenin-responsive transcription (CRT; IC50 = 40.3 nM)
|
|---|---|
| 体外研究 (In Vitro) |
除了影响 TCF-β-cat 反应外,iCRT 14 还能够阻碍 TCF 的 DNA 结合 [1]。尽管仍低于 iCRT 3,但 iCRT 14(10、25、50 μM)可有效且呈时间和剂量依赖性地减少 BT-549 细胞的生长 [2]。
|
| 体内研究 (In Vivo) |
在 HCT116 异种移植物中,iCRT 14(50 mg/kg,腹腔注射)显着降低 CycD1(肿瘤肿胀)[1]。
|
| 酶活实验 |
热稳定性分析。[1]
在室温下,在不同浓度的iCRTs(如iCRT-14)和0.5×SYPRO Orange的存在下,将纯化的β-cat-His和TCF4-N-GST以1:2的摩尔比在1×PBS中混合。使用LightCycler 480系统在96孔板中以0.06°C/s的升温速率将样品从20°C加热到95°C。在加热阶段,以0.1s的间隔采集荧光读数。通过绘制荧光强度读数的负导数与温度的关系来计算熔化温度。负导数曲线上的拐点被认为是熔化温度(Tm)。 |
| 细胞实验 |
对于每种细胞系的细胞增殖测定,细胞用DMSO作为载体或不同浓度的每种Wnt抑制剂处理:iCRT-3(25、50、75μM)、iCRT-5(50、100、200μM),iCRT-14(10、25、50μM);IWP-4(1、2.5、5μM)和XAV-939(5、10μM)。对于SOX4敲除的BT549细胞的细胞增殖、迁移和侵袭试验,用DMSO或25μM iCRT-3处理细胞。CIM板16的上腔涂有Matrigel(1:40稀释),用于细胞侵袭试验。此外,在实验时用50μM染料木素和25μM iCRT-3处理6天的SOX4敲除的BT-549细胞中测量了细胞增殖。每个样品都进行了三次分析,并进行了三个独立的实验。细胞增殖试验进行48小时,细胞迁移和侵袭实验进行24小时。RTCA软件包1.2计算了每个样本的细胞指数值,用于测量电阻抗的相对变化,以表示细胞形态、粘附或存活率。[2]
将细胞以20000个细胞/孔的速度接种到96孔板中。孵育过夜后,用DMSO或每种Wnt抑制剂(iCRT-3,75μM;iCRT-5,200μM;iCRT-14,50μM;IWP-4,5μM和XAV-939,10μM)处理细胞48小时。根据制造商的说明,使用Cell Titer Glo发光细胞存活率测定试剂盒测定细胞存活率。使用FLUOstar微孔板读数器测量发光。所有治疗均进行三次,每次实验重复三次[2]。 |
| 参考文献 |
|
| 其他信息 |
Misregulated β-catenin responsive transcription (CRT) has been implicated in the genesis of various malignancies, including colorectal carcinomas, and it is a key therapeutic target in combating various cancers. Despite significant effort, successful clinical implementation of CRT inhibitory therapeutics remains a challenging goal. This is, in part, because of the challenge of identifying inhibitory compounds that specifically modulate the nuclear transcriptional activity of β-catenin while not affecting its cytoskeletal function in stabilizing adherens junctions at the cell membrane. Here, we report an RNAi-based modifier screening strategy for the identification of CRT inhibitors. Our data provide support for the specificity of these inhibitory compounds in antagonizing the transcriptional function of nuclear β-catenin. We show that these inhibitors efficiently block Wnt/β-catenin-induced target genes and phenotypes in various mammalian and cancer cell lines. Importantly, these Wnt inhibitors are specifically cytotoxic to human colon tumor biopsy cultures as well as colon cancer cell lines that exhibit deregulated Wnt signaling.
[1]
Background: Triple-negative breast cancer (TNBC) is an aggressive clinical subtype of breast cancer that is characterized by the lack of estrogen receptor (ER) and progesterone receptor (PR) expression as well as human epidermal growth factor receptor 2 (HER2) overexpression. The TNBC subtype constitutes approximately 10%-20% of all breast cancers, but has no effective molecular targeted therapies. Previous meta-analysis of gene expression profiles of 587 TNBC cases from 21 studies demonstrated high expression of Wnt signaling pathway-associated genes in basal-like 2 and mesenchymal subtypes of TNBC. In this study, we investigated the potential of Wnt pathway inhibitors in effective treatment of TNBC. Methods: Activation of Wnt pathway was assessed in four TNBC cell lines (BT-549, MDA-MB-231, HCC-1143 and HCC-1937), and the ER+ cell line MCF-7 using confocal microscopy and Western blot analysis of pathway components. Effectiveness of five different Wnt pathway inhibitors (iCRT-3, iCRT-5, iCRT-14, IWP-4 and XAV-939) on cell proliferation and apoptosis were tested in vitro. The inhibitory effects of iCRT-3 on canonical Wnt signaling in TNBC was evaluated by quantitative real-time RT-PCR analysis of Axin2 and dual-luciferase reporter assays. The effects of shRNA knockdown of SOX4 in combination with iCRT-3 and/or genistein treatments on cell proliferation, migration and invasion on BT-549 cells were also evaluated. Results: Immunofluorescence staining of β-catenin in TNBC cell lines showed both nuclear and cytoplasmic localization, indicating activation of Wnt pathway in TNBC cells. iCRT-3 was the most effective compound for inhibiting proliferation and antagonizing Wnt signaling in TNBC cells. In addition, treatment with iCRT-3 resulted in increased apoptosis in vitro. Knockdown of the Wnt pathway transcription factor, SOX4 in triple negative BT-549 cells resulted in decreased cell proliferation and migration, and combination treatment of iCRT-3 with SOX4 knockdown had a synergistic effect on inhibition of cell proliferation and induction of apoptosis. Conclusions: These data suggest that targeting SOX4 and/or the Wnt pathway could have therapeutic benefit for TNBC patients.[2] |
| 分子式 |
C21H17N3O2S
|
|---|---|
| 分子量 |
375.44358
|
| 精确质量 |
375.104
|
| 元素分析 |
C, 67.18; H, 4.56; N, 11.19; O, 8.52; S, 8.54
|
| CAS号 |
677331-12-3
|
| 相关CAS号 |
901751-47-1(iCRT3); 18623-44-4 (iCRT5)
|
| PubChem CID |
5967294
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| LogP |
4.795
|
| tPSA |
80.5
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
617
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC1=CC(=C(N1C2=CN=CC=C2)C)/C=C\3/C(=O)N(C(=O)S3)C4=CC=CC=C4
|
| InChi Key |
NCSHZXNGQYSKLR-XDHOZWIPSA-N
|
| InChi Code |
InChI=1S/C21H17N3O2S/c1-14-11-16(15(2)23(14)18-9-6-10-22-13-18)12-19-20(25)24(21(26)27-19)17-7-4-3-5-8-17/h3-13H,1-2H3/b19-12+
|
| 化学名 |
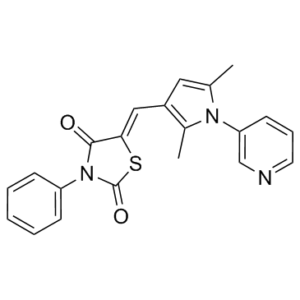
5-[[2,5-dimethyl-1-(3-pyridinyl)-1H-pyrrol-3-yl]methylene]-3-phenyl-2,4-thiazolidinedione
|
| 别名 |
iCRT14; iCRT-14; 677331-12-3; iCRT-14; iCRT14; (5Z)-5-[(2,5-dimethyl-1-pyridin-3-ylpyrrol-3-yl)methylidene]-3-phenyl-1,3-thiazolidine-2,4-dione; CHEMBL3589010; (5Z)-5-{[2,5-Dimethyl-1-(3-pyridinyl)-1H-pyrrol-3-yl]methylene}-3-phenyl-1,3-thiazolidine-2,4-dione; (5Z)-5-{[2,5-dimethyl-1-(pyridin-3-yl)-1H-pyrrol-3-yl]methylidene}-3-phenyl-1,3-thiazolidine-2,4-dione; iCRT 14
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 29 mg/mL (~77.24 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.66 mM) (饱和度未知) in 10% DMSO + 40% PEG300 +5% Tween-80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80+,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6635 mL | 13.3177 mL | 26.6354 mL | |
| 5 mM | 0.5327 mL | 2.6635 mL | 5.3271 mL | |
| 10 mM | 0.2664 mL | 1.3318 mL | 2.6635 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
 iCRT-14 effectively inhibits cell proliferation in BT-549 cells in a dose- and time-dependent manner.J Transl Med.2013 Nov 4;11:280. |
|---|
 (A) Primary screen. dsRNA-mediated knockdown of Axin results in cytoplasmic stabilization of β-cat, which, on translocation to the nucleus, results in activation of the β-cat responsive dTF12 reporter.Proc Natl Acad Sci U S A.2011 Apr 12;108(15):5954-63. |
 (A) Effect of candidate compounds on the interaction of purified β-cat-His and GST-TCF4. iCRT3, -5, and -14 show a significant inhibitory effect on these interactions compared with nontreated (NT) and DMSO-treated binding reactions.Bottomshows comparable amounts of GST-TCF4 being pulled down.Proc Natl Acad Sci U S A.2011 Apr 12;108(15):5954-63 |