| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 靶点 |
NMDA receptor;
|
|---|---|
| 体外研究 (In Vitro) |
由 G 蛋白激活的内向整流 K+ 通道 (GIRK)[1]。 通过电压箝法记录克隆NR1和NR2亚基rna表达异源NMDA受体的爪蟾卵母细胞,研究了非典型n -甲基- d -天冬氨酸(NMDA)受体拮抗剂Ifenprodil的作用。在-70 mV电压箝位的卵母细胞中,Ifenprodil对NR1A/NR2B受体的nmda诱导电流具有高亲和力抑制作用(IC50 = 0.34微米)。NR1A/NR2A受体对伊芬普罗地尔的亲和力(IC50 = 146 microM)比NR1A/NR2B受体低400倍。低浓度的伊芬普罗地尔作用于NR1A/NR2B受体时的抑制起效率明显慢于高浓度的伊芬普罗地尔作用于NR1A/NR2A受体时的抑制起效率。Ifenprodil/伊芬普罗地尔阻断NR1A/NR2B受体的开始和恢复不具有活性依赖性。低浓度的伊芬普罗地尔对NR1A/NR2B受体的抑制作用不依赖于电压。相反,高浓度伊芬普罗地尔对NR1A/NR2A受体的抑制作用部分依赖于电压,并且在超极化膜电位处比在去极化膜电位处对nmda诱导电流的抑制更大。在伊芬地尔的作用下,NMDA电流的逆转电位没有改变。伊芬普罗地尔可能作为NR1A/NR2A受体的弱开放通道阻滞剂。100微米的非芬普罗地尔对NR1A/NR2A受体的抑制程度不因细胞外甘氨酸浓度的变化而改变。然而,增加甘氨酸浓度可降低1 μ m非芬地尔对NR1A/NR2B受体的抑制作用。因此,伊芬普罗地尔作用于NR1A/NR2B受体的部分机制可能涉及对甘氨酸作用的非竞争性拮抗。这些结果表明,对爪蟾卵母细胞中表达的NR1A/NR2B和NR1A/NR2A受体,伊芬地尔的作用机制和拮抗剂的效力不同。[1]
G蛋白激活的内向纠偏K+通道(GIRK,也称为Kir3)受多种G蛋白偶联受体调节。GIRK通道的激活在降低大多数大脑区域的神经元兴奋性和心率方面起着重要作用。Ifenprodil是一种临床使用的脑血管扩张剂,可与几种受体相互作用,如α - 1肾上腺素能受体、n -甲基- d -天冬氨酸受体、血清素受体和西格玛受体。然而,各种临床相关作用的分子机制仍有待阐明。在这里,我们通过非洲爪蟾卵细胞表达测定来检测Ifenprodil/伊芬普罗地尔对GIRK通道的影响。在注射了GIRK1/GIRK2、GIRK2或GIRK1/GIRK4亚基mrna的卵母细胞中,伊芬prodil通过基础GIRK活性可逆地降低了内向电流。这种抑制作用是浓度依赖性的,但与电压和时间无关,这表明伊芬普罗地尔可能不是通道的开放通道阻滞剂。相反,其他Kir亚家族的Kir1.1和Kir2.1通道对伊芬普罗地尔不敏感。此外,克隆的kappa-阿片受体激活的GIRK电流反应同样被伊芬普罗地尔抑制。在细胞内施用伊芬普罗地尔时,没有观察到其抑制作用,也不受细胞外pH的影响,pH改变了未带电的质子化伊芬普罗地尔的比例,表明其作用于细胞外侧。在异丙地尔的存在下,乙醇诱导的GIRK电流也有所减弱。我们的研究结果表明,在亚微摩尔浓度或更高浓度下,伊芬地尔直接抑制GIRK通道可能有助于其一些治疗效果和不良副作用[2]。 在基于CPE的抗病毒试验中,Ifenprodil与nylidrin相当,PR8和HK的EC50值分别为6.6和16.9μM(表1)。盐酸克伦特罗仅对PR8具有强效活性,EC50值为9.4μM,而拉贝洛尔和依利地尔仅对HK具有微弱疗效,EC50值更高,高于44.0μM。相比之下,利多君、非诺特罗和班布特罗对任何病毒株都没有抗病毒活性。在进一步的实验中,我们测试了抑制化合物,甚至部分包括nylidrin、Ifenprodil、拉贝洛尔、依利普地尔和克伦特罗,以对抗其他甲型和乙型流感病毒(表2)。Nylidrin、Ifenprodil和克伦特罗对甲型H1N1流感病毒株一直有效,但它们的疗效在H3N2病毒株之间有所不同,在乙型流感病毒感染的细胞中没有抗病毒活性。正如预期的那样,剩下的两种化合物拉贝洛尔和依利普地尔对这些病毒株几乎没有影响,除了A/Brisbane/59/2007(H1N1),它对依利普迪尔部分敏感(EC50,53.1μM)。这些结果表明,nylidrin及其类似物Ifenprodil和克伦特罗在亚毒性浓度下可以可靠地抑制甲型H1N1流感病毒的感染[4]。 Ifenprodil与NMDA受体的相互作用降低了离子通道的开放状态,并抑制了Ca2+离子的流入。关于这一机制,依非普利具有神经保护、抗惊厥和镇痛作用。ifenprodil的结合位点最初被认为位于GluN2B亚基的氨基末端结构域(ATD)。后来,它可以在GluN1和GluN2B亚基之间的表面发现。根据第一个开发的配体,它被称为“ifenprodil结合位点”,可以与受体的不同其他结合位点相互作用。伊芬普利的NMDA亲和力非常高(IC50=13.3 nM,Ki=10 nM),但其选择性相当差[5]。 |
| 体内研究 (In Vivo) |
我们发现,无论是Ifenprodil还是布比卡因,都能剂量依赖性地产生运动功能和伤害感受的脊髓阻滞。在ED50的基础上,异环磷酰胺的效力为0.42(0.38-0.46)μmol;0.40(0.36-0.44)μmol)与布比卡因0.38(0.36-0.40)μmol相等(p>0.05);0.35(0.32-0.38)μmol)分别影响运动功能和伤害感受。在等麻醉剂量(ED25、ED50和ED75)下,在运动功能和伤害感受方面,Ifenprodil产生的持续时间大于布比卡因(差异p<0.05)。此外,伊芬普地尔和布比卡因的感觉阻滞持续时间均长于运动阻滞(差异p<0.05)。
结论:结果数据表明,在脊髓麻醉中,Ifenprodil具有剂量依赖性的局部麻醉作用。Ifenprodil显示出比运动阻滞更具感觉选择性的作用持续时间,而Ifenprodi的麻醉持续时间明显长于布比卡因。[3]
本研究的结果首次表明,鞘内注射Ifenprodil可引发运动功能和伤害性感觉的脊髓阻滞。Ifenprodil的脊髓麻醉效果与长效局部麻醉剂布比卡因相当。Ifenprodil和布比卡因显示出比运动阻滞更长的伤害性/感觉阻滞持续时间。在等麻醉剂量(ED25、ED50和ED75)下,Ifenprodil的脊髓麻醉持续时间大于。.. 我们的临床前数据显示,鞘内注射Ifenprodil在脊髓麻醉中产生了剂量依赖性的局部麻醉作用。Ifenprodil在脊髓麻醉中的效力与布比卡因相似,而Ifenprodil的脊髓麻醉持续时间大于布比卡因。此外,Ifenprodil和布比卡因表现出明显的感觉特异性过运动阻滞。Ifenprodil的神经阻滞值得进一步测试[3]。 体内代谢产物的鉴定[5] 对于体内代谢物,一只大鼠在腹腔注射20mgIfenprodil/kg大鼠后,分三个时期(0-8h、8-24h和24-48h)收集48小时的尿液。鉴定的代谢物如图7所示。仅在体内发现了三种代谢物(11、12和13)。代谢物11是邻羟基化、甲基化和葡萄糖醛酸化的结果。区域异构体葡糖苷酸12a和12b具有与8相同的质量(m/z 518),但显示出不同的裂解模式(见支持信息)。它们是从羟基化代谢物4开始产生的,这些代谢物还被葡萄糖醛酸化。与代谢物4类似的裂解模式允许识别相应的代谢物。此外,观察到两种具有额外O原子的代谢物13,但裂解模式无法明确指定O原子的位置。在加入PAPS后,在体外系统中鉴定出的硫酸盐10在大鼠尿液中没有检测到。然而,这种代谢物是否在样本尿液中或在储存过程中没有形成或分解仍有待阐明。 |
| 酶活实验 |
Microsomal incubations (phase I) (第一阶段)[5]
将Ifenprodil(235μg)溶解在含有氯化镁(100μL,45.5mM)的磷酸盐缓冲液(250μL,pH 7.4,0.1M)中。在加入150μL微粒体制剂(7mg蛋白质/mL)和5mg NADPH/H+后,将混合物在室温下在摇床中孵育。120分钟后,通过加入等体积的冷乙腈(-20°C)终止孵育。将混合物在冰浴中储存10分钟。随后,将样品在13000 rpm下离心8分钟,并在不进行进一步样品预处理的情况下通过HPLC-MS分析所得上清液。 Ifenprodil代谢降解率[5] 在校准研究中,将[strong>Ifenprodil(117.5μg)溶解在磷酸钠缓冲液(125μL,pH 7.4,0.1 M)中。加入氯化镁(50μL,45.5 mM)和大鼠肝微粒体悬浮液(100μL)。用100、80、60、40、20和0%的母体伊芬普利浓度(117.5μg)进行校准,用磷酸钠缓冲液稀释以获得所需浓度。120分钟后,通过加入冷CH3CN(500μL,-20°C)停止反应,并加入内标eliprodil(62.75μg,溶解在32.5μL CH3CN中)。将混合物在冰浴中储存10分钟,随后离心(13000rpm,8分钟)。用CH3CN/H2O(1/1)将上清液稀释1:40,并注入HPLC系统1。每个校准步骤进行三次。手动整合得到的TIC(m/z=326.2和m/z=348.15)。使用Microsoft Excel 2010计算eliprodil/Ifenprodil比值和所得校准曲线。 用溶解在磷酸钠缓冲液(125μL,pH 7.4,0.1 M)中的伊芬普利(117.5μg)进行代谢降解。加入氯化镁(50μL,45.5 mM)、大鼠肝微粒体(100μL)、NADPH(2.5 mg)和UDPGA(2 mg)。将该反应混合物摇动15、30、60、90和120分钟。然后,通过加入冷乙腈(500μL,-20°C)和依利脯啶(62.75μg,溶解在32.5μL CH3CN中)停止反应。将混合物在冰浴中储存10分钟,随后离心(13000rpm,8分钟)。用乙腈/H2O(1/1)将上清液稀释1:40,并注入HPLC系统1。每一步都进行了三次。由此产生的TIC(m/z 326.2和m/z 348.15)是手动集成的。使用Microsoft Excel 2010计算依普罗地尔/Ifenprodil比率和由此产生的依普罗地尔量。 |
| 细胞实验 |
为了检查细胞内Ifenprodil的作用,23 nl为10 mM ifenprodil或30 mM lidocaine N-ethyl bromide (QX-314) 溶解在蒸馏水中的mM利多卡因N-乙基溴(QX-314)通过使用Nanoliter注射器进行压力注射的额外移液管施用于卵母细胞,如前所述(Kobayashi等人,2003),然后连续记录卵母细胞电流约30-40 由于使用的爪蟾卵母细胞体积约为1 μl,细胞内Ifenprodil或QX-314的浓度被推测为∼225或∤674 μM。使用KaleidaGraph将数据拟合到标准逻辑斯谛方程中,以分析浓度-反应关系。EC50值,即产生该药物最大电流反应50%的药物浓度;IC25和IC50值,分别是将控制电流反应降低25%和50%的药物浓度;Hill系数(nH)由浓度-响应关系获得。[2]
化合物和细胞病变效应(CPE)减少试验[4] 使用了试验化合物盐酸尼利德林(~95%)、Ifenprodil(+)-酒石酸盐(≥98%)、盐酸拉贝洛尔(≥98%)、盐酸利托君(99.6%)、氢溴酸非诺特罗(≥98 0%)、依利普地尔(≥98%.)、盐酸克伦特罗(≥95%)和盐酸班布特罗(≥98%.)、M2抑制剂盐酸金刚烷胺(AMT;≥98%)和聚合酶抑制剂利巴韦林(RBV;≥98%.)、奥司他韦羧酸盐(OSV-C;≥98%。 免疫测定[4] 为了检测病毒蛋白,如前所述进行了蛋白质印迹分析。在nylidrin、OSV-C或RBV存在下,在35°C下用MOI为0.001的PR8病毒感染6孔板中生长至100%融合的MDCK细胞24小时。通过免疫印迹法检测病毒蛋白NP、HA和M1,分别检测相应的抗体:小鼠抗NP、兔抗HA2 和小鼠抗M1。第二抗体是辣根过氧化物酶(HRP)偶联的山羊抗小鼠或抗兔IgG。细胞β-肌动蛋白被用作负载对照。 菌斑检测[4] PR8感染的MDCK细胞被模拟处理或用单独的化合物处理。在35°C下24小时后,收集培养上清液以制备10倍的连续稀释液,用于感染接种在48孔板中的幼稚MDCK细胞1小时。PBS洗涤后,将其在33°C的覆盖培养基(含0.6%羧甲基纤维素和2µg/mL TPCK胰蛋白酶的MEM)中孵育三天。通过结晶紫染色计数斑块。 对于添加时间实验,在有或没有nylidrin的情况下,在4°C下用PR8(MOI,0.001)感染48孔板中的MDCK细胞1小时。用PBS洗涤以去除化学物质和未吸附的病毒后,在1、2和4小时p.i.(−)给药nylidrin。将表没食子儿茶素没食子酸酯用作阻断病毒进入的对照。随后,所有样品用PBS以5小时p.i.洗涤,然后在覆盖培养基下孵育三天,然后进行结晶紫染色。 共聚焦显微镜[4] 在荧光显微镜下,将PR8感染的MDCK细胞在有或没有nylidrin的情况下在37°C下孵育5小时。固定和渗透后,使用抗NP抗体和Alexa Fluor 488偶联的山羊抗小鼠IgG观察病毒NP;用4′,6-二脒基-2-苯基吲哚对核DNA进行复染。图像在蔡司LSM 700共聚焦显微镜上捕获,并使用ZEN软件进行分析。 为了研究病毒NP与细胞Rab5或Rab7的共定位,将A549细胞接种在4孔载玻片中(每孔4×104个细胞)。第二天,根据制造商的说明,使用脂质体2000用0.4μg pEGFP-Rab5或pEGFP-Rab 7转染细胞。24小时后,在4°C下,在100μM nylidrin存在或不存在的情况下,模拟感染或以10的MOI感染PR8 30分钟,然后在37°C下再感染4小时。使用上述相同的一抗,但用Alexa Fluor 633偶联的二抗探测病毒NP蛋白。 |
| 动物实验 |
For isolating liver microsomes and for in vivo metabolism studies of Ifenprodil, Wistar rats weighing 200–320 g from a local strain were used. [5]
In vivo metabolism studies of Ifenprodil [5] Ifenprodil was dissolved in 0.9% sterile saline solution. A single dose of 6.2 mg Ifenprodil tartrate was administered i.p. to one female rat (310 g, Wistar rat), which results in a dose of 20 mg/kg. A dose of 20 mg/kg ifenprodil was chosen, since traxoprodil, which has similar structure and pharmacological properties, was used in previous experiments in a comparable amount [12]. The rat was housed individually in a metabolism cage with water and powdered feed ad libitum. Urine was collected for 48 h in three intervals of 0–8 h, 8–24 h and 24–48 h after i.p. application of ifenprodil. The volumes of urine samples were recorded and the samples were stored at −20 °C until analysis or directly used. Extraction was carried out with RP-18 SPE cartridges, which were pre-conditioned with 1 mL H2O and 1 mL EtOH. After application of the urine samples, the cartridge was rinsed with 10 mL of H2O. Afterwards, Ifenprodil and the corresponding metabolites were eluted with 10 mL of EtOH. The solvent was removed under reduced pressure. The resulting solid was dissolved in CH3CN:H2O 1:1 and analyzed with the different HPLC systems. The aim of the study was to compare the proposed spinal anesthetic effect of Ifenprodil, an a1 adrenergic receptor antagonist, with that of the long-acting local anesthetic bupivacaine. Methods: After intrathecally injecting the rats with five different doses of each drug, the dose-response curves of Ifenprodil and bupivacaine were constructed to obtain the 50% effective dose (ED50). The spinal blockades of motor function and nociception of Ifenprodil were compared with that of bupivacaine.[3] Male Sprague–Dawley rats (300–350 g) were used. Intrathecal Ifenprodil caused significant motor or nociceptive blockade. Intrathecal Ifenprodil, as well as the long-lasting local anesthetic bupivacaine produced a dose-dependent local anesthetic effect in spinal anesthesia in rats (Fig. 1). The ED25s, ED50s, and ED75s of Ifenprodil and bupivacaine are presented in Table 1. On the ED50 basis, the potency of Ifenprodil in motor function and nociception was comparable to that of bupivacaine (Table 1, p > 0.05). [3] |
| 药代性质 (ADME/PK) |
The NMDA receptor antagonist Ifenprodil is an important lead structure for developing new GluN2B selective NMDA receptor antagonists. Ifenprodil itself has a high affinity to the GluN2B subunit but a poor selectivity for the NMDA receptor. This aspect and the fast biotransformation are the major drawbacks of ifenprodil. In order to optimize the development of new and more selective GluN2B (NMDA) receptor antagonists, the identification of the main metabolic pathways of ifenprodil is necessary. Herein the in vitro and in vivo phase I and phase II metabolites of Ifenprodil were generated and analyzed via LC-MS(n) experiments. In vitro experiments were carried out with rat liver microsomes and various co-factors to generate phase I and phase II metabolites. The application of ifenprodil to a rat and the analysis of its urine led to the identification of diverse formed in vivo metabolites. The phenol represents the metabolically most labile structural element since glucuronide 7 and 8 appeared as main metabolites. [5]
It has been reported that the bioavailability of Ifenprodil is rather low. The maximum plasma level in humans was found after ∼30 min [9]. However the biotransformation of ifenprodil and the structure of its metabolites have not been described so far. Therefore, identification of the metabolically labile positions of ifenprodil might contribute to the development of metabolically more stable drugs interacting with the ifenprodil binding site. Herein we report on our studies of phase I and phase II metabolism of ifenprodil in vitro and in vivo. [5] Fragmentation of Ifenprodil [5] MSn experiments of ifenprodil revealed a fragmentation pattern which can be considered quite typical for the structure of ifenprodil (Fig. 1). The first fragmentation step is the dehydration of the secondary alcohol (m/z 308.2003). Further fragmentation leads to a fragment with m/z 293.1752, which can be explained by the loss of a methyl group. Another important fragmentation of Ifenprodil is the cleavage of the Csingle bondN (piperidine) bond leading to the formation of the benzylpiperidine moiety with m/z 176.1424. Release of the tropylium ion (m/z 91.0511) further proves this fragment. This fragmentation pattern of the parent compound ifenprodil represents the basis for the interpretation of the fragmentation studies of the produced metabolites, providing information about the structures of the formed metabolites. Identification and fragmentation of the phase I metabolites in vitro [5] Phase I transformation led to several metabolites including two N-dealkylation products and several M+O metabolites (Fig. 2, supporting information). The N-dealkylated metabolites were identified as 4-benzyl-piperidine (1) and oxidized 4-benzyl-piperidin-2-on (2). The structures of the piperidine metabolites 1 and 2 were identified by fragmentation experiments. The main fragment of both metabolites is the tropylium ion with an exact mass of m/z 91.0510 and m/z 91.0518, respectively (see sup. inf.). Furthermore, six phase I metabolites were identified with m/z 342 [M+O+H]+, caused by introduction of an additional O-atom (Fig. 3a). The metabolites were analyzed with MSn experiments. According to this analysis oxidation took place at the piperidine ring (3), in ortho-, meta- and para-position of the phenyl moiety (4a–4c), in ortho position of the phenol (5), and at the N-atom forming the N-oxide (6, Fig. 3b). The three metabolites 4a–4c showed identical fragmentation pattern, indicating very similar structures. The hydroxy group of the monohydroxylated metabolite 3 was assigned to the piperidine heterocycle. The first hint was the fragment m/z 192. This mass is 16 amu higher than the mass of the corresponding fragment of the parent compound. The subsequent fragmentation led to a fragment with m/z 174, which represents the dehydrated benzylpiperidine. These fragments prove the position of the hydroxy group because the fragment m/z 174 is only possible when the hydroxy group was located in the piperidine moiety (Fig. 4). The N-oxide 6 was identified by the fragments m/z 192.1358 (oxidized benzylpiperidine) and m/z 174 [benzylpiperidine+O+H–H2O]+. Thus the additional O-atom can only be localized at the benzylpiperidine part of Ifenprodil. The slightly increased retention time (10.8 min) compared to Ifenprodil (9.4 min) indicates an N-oxide. N-Oxides are known to have longer retention times then their parent compounds due to their slightly increased lipophilicity. Phase II metabolites found in vitro [5] In phase II reactions Ifenprodil can theoretically be converted into glucuronides, sulfates and methylated catechol derivates as described for traxoprodil. β-Glucuronides and sulfates could be formed directly by reaction of the hydroxy groups present in ifenprodil. Additionally, metabolites from phase I reactions can be conjugated in phase II reactions. Methylated catechol derivates are only possible after formation of catechol derivatives by hydroxylation in o-position of the phenol (compare metabolite 5). The glucuronidation was investigated in vitro by addition of UDPGA to the microsomal incubation mixture without addition of NADPH/H+. The observed signal for the glucuronide 7 [M+Glu+H]+ was recorded in positive and negative ion mode. Fragmentation of the β-glucuronide metabolite 7 in positive mode showed two main fragmentation pathways. Similar to Ifenprodil and its derivatives, loss of water was observed (m/z 484.2346 in positive ion mode). On the other hand glucuronic acid was cleaved off resulting in the fragment m/z 326.2134 ([ifenprodil+H]+). Both fragmentations took place in parallel and led to the same further fragment m/z 308.2040 (see SI). In the negative ion mode analogous fragments were observed. Additionally, the fragments of glucuronic acid were identified in negative ion mode. The fragment m/z 193 represents the glucuronate anion, whilst m/z 175 corresponds to a dehydrated glucuronic acid. Subsequent loss of water and CO2 led to fragment m/z 113 which is characteristic for glucuronides (Fig. 5). The incubation of Ifenprodil with NADPH/H+ and UDPGA resulted in the additional glucuronide 8 (Fig. 2), which occurred after glucuronidation of the catechol 5, which was produced during phase I biotransformation. The same catechol 5 could also be methylated by catechol O-methyl transferases. The addition of S-adenosyl methionine (SAM) and NADPH/H+ to the incubation mixture resulted in the formation of a compound with m/z 356.1965, corresponding to the mass of the methylated catechol 9. The fragmentation of 9 (Fig. 6) proceeded analogously to the fragmentation of ifenprodil (see Fig. 1). The formation of the fragment m/z 176.1444 corresponding to the unsubstituted benzylpiperidine dearly proves that the methoxylation had occurred at the phenol moiety and is not the result of a methoxylation of the benzyl moiety. Moreover, the mass of the fragments m/z 338.2129, 163 and 137 are 30 amu higher than the mass of the corresponding ifenprodil fragments. Identification of metabolites formed in vivo [5] For in vivo metabolites the urine of one rat was collected for 48 h in three periods (0–8 h, 8–24 h and 24–48 h) after i.p. injection of 20 mg Ifenprodil/kg rat. The identified metabolites are shown in Fig. 7. Three types of metabolites (11, 12 and 13) were found only in vivo. Metabolite 11 is the result of o-hydroxylation, methylation and glucuronidation. The regioisomeric glucuronides 12a and 12b have the same mass (m/z 518) as 8, but show a different fragmentation pattern (see supporting information). They were generated starting from hydroxylated metabolites 4 which were additionally glucuronidated. The fragmentation patterns comparable to metabolites 4 allowed the identification of the corresponding metabolites. Furthermore, two metabolites 13 with an additional O-atom were observed, but the fragmentation pattern did not allow the unequivocal assignment of the position of the O-atom. The sulfate 10, which has been identified in the in vitro system after addition of PAPS, was not detected in the urine of the rat. However, whether this metabolite was not formed or was decomposed in the sample urine or during storage remains to be elucidated. Ifenprodil was excreted rapidly during the first period (0–8 h). In this period, ifenprodil is one of the main compounds, together with the glucuronides 7 and 11, assuming comparable ionization factors of all compounds. Glucuronidation was observed as the main metabolic pathway within the first two periods (0–8 h, 8–24 h). The detection of ifenprodil even in the last period of urine collection (24–48 h) indicates the unmodified excretion of ifenprodil even after 24 h (Fig. 8). Metabolic stability of Ifenprodil [5] In in vitro experiments the metabolic stability of ifenprodil in the presence of rat liver microsomes, NADPH/H+ and UDPGA was determined. These co-factors were chosen to generate the main metabolites observed in vivo. For the exact quantification of ifenprodil, a calibration curve was recorded first. Ifenprodil was incubated in different amounts (20, 40, 60, 80 and 100% of the amount used for the incubation with microsomes) with the reaction mixture without NADPH/H+. Additionally, eliprodil was added as internal standard (IS). The ratio ifenprodil/IS was plotted against the employed Ifenprodil amount, which resulted in a good regression coefficient (see supporting information). The ifenprodil concentration in the metabolic active mixture was determined after six periods of incubation up to 120 min. Unexpectedly, incubation with NADPH/H+ and UDPGA resulted in increasing Ifenprodil concentrations after 60 min. This result was explained with fast decomposition of the glucuronide 7, which leads to regeneration of ifenprodil. This theory was confirmed by incubation of ifenprodil only with NADPH/H+ without UDPGA which resulted in continuously decreasing ifenprodil amounts (Fig. 9). After an incubation period of 60 min with both co-factors, 86 ± 2% of ifenprodil remained to be detected. Generation of only phase I metabolites reduced the amount of ifenprodil to 92.8 ± 2% after 60 min. Thus, the main biotransformation of Ifenprodil was caused by glucuronidation reactions. This observation corresponds well with the in vivo experiments, leading to glucuronides 7 and 8 as main metabolites, too. The biotransformation of Ifenprodil, an important lead compound for the development of potent and selective GluN2B selective NMDA receptor antagonists, was analyzed. In in vitro experiments, N-dealkylation, hydroxylation of both aromatic rings and the piperidine moiety were identified as possible reactions. In phase II experiments, glucuronidation of the phenol was observed. Additionally, after incubation with the corresponding co-factors SAM und PAPS, methoxylated and sulfated metabolites were detected. The analysis of a rat urine sample led to the identification of glucuronides, hydroxylated and methoxylated metabolites. The glucuronide 7 was identified as main metabolite. Altogether, the phenol of Ifenprodil is the main structural element susceptible for biotransformation. Especially glucuronidation of the OH group was identified as the main metabolic pathway in vitro and in vivo. These results clearly indicate that the phenol should be replaced bioisosterically in order to obtain metabolically more stable GluN2B selective NMDA receptor antagonists. [5] |
| 毒性/毒理 (Toxicokinetics/TK) |
The interaction of Ifenprodil with other receptors in the CNS (α1, 5-HT, σ1, σ2 receptor), leads to undesired side effects, e.g. impaired motor function and reduced blood pressure. Nevertheless, ifenprodil serves as an important lead structure for the rational design of novel GluN2B selective antagonists bearing the potential of becoming drugs for life-threatening CNS diseases [5].
|
| 参考文献 |
|
| 其他信息 |
4-[1-hydroxy-2-[4-(phenylmethyl)-1-piperidinyl]propyl]phenol is a member of piperidines.
N-methyl-D-aspartate (NMDA) receptors (NMDARs) are members of the ionotropic glutamate receptor family, with key roles in brain development and neurological function. NMDARs are heterotetramers that typically involve a dimer of dimers of both GluN1 and GluN2A-D subunits, with each subunit itself composed of an N-terminal domain (NTD), a ligand-binding domain (LBD), a transmembrane domain, and a C-terminal cytoplasmic domain. Binding at the LBD of the agonists glycine (or D-serine) to the GluN1 subunits and of glutamate to the GluN2 subunits is a regulatory mechanism for channel activation. In addition, allosteric modulators are known to bind at the NTDs and form another layer of regulation. One such allosteric regulator is Ifenprodil, which was first shown to bind the NMDARs in the 1990s, and specifically to those NMDARs containing the GluN2B subunit. Further studies elucidated that ifenprodil binds strongly at the inter-subunit interface of adjacent GluN1 and GluN2B NTDs, where it acts as a non-competitive antagonist. Although ifenprodil has received considerable interest in its potential neuromodulatory activities in psychiatric conditions, including dependency and depression, it has also been shown to have an immunomodulatory effect. In an unbiased screen for compounds capable of reducing cell death induced by infection with the influenza strain H5N1, ifenprodil was found to have a protective effect against H5N1-induced lung damage, in part through its ability to alleviate the H5N1-induced cytokine storm and reduce pulmonary infiltration of neutrophils, natural killer cells, and T cells. Ifenprodil is being investigated for its potential utility in treating COVID-19 in an ongoing phase 2b/3 clinical trial (NCT04382924). Ifenprodil is an orally bioavailable, N-methyl-D-aspartate (NMDA) receptor antagonist, with potential central nervous system (CNS) stimulating, neuroprotective, anti-inflammatory and anti-fibrotic activities. Upon administration, ifenprodil targets, binds to and inhibits glutamanergic NMDA receptors (NMDARs), specifically the glycine-binding NMDA receptor subunit 1 (GluN1) and 2 (glutamate-binding NMDA receptor subunit 2; NMDA-type subunit 2B; GluN2B), thereby preventing NMDAR signaling. This inhibits neuronal excitotoxicity, and thereby potentially enhancing cognitive function. Additionally, ifenprodil inhibits G protein-coupled inwardly-rectifying potassium (GIRK) channels, and interacts with alpha1 adrenergic, serotonin, and activates sigma receptors. Ifenprodil exerts its anti-inflammatory effect through its effect on NMDA and possibly sigma-1 receptors. Although the exact mechanism has not fully been elucidated, this agent reduces the infiltration of neutrophils and T-cells into the lungs and prevents the release of pro-inflammatory cytokines. This may result in the reduction of the lung inflammatory response, inhibit fibrosis in the lungs and may reduce the severity of cough. NMDA receptors are multimeric ionotropic glutamate receptors composed of four subunits and are expressed on various cells and organs, such as in the brain, lungs, and on T-cells and neutrophils. Characteristics of GIRK Channel Inhibition by Ifenprodil [2] The present study demonstrated that Ifenprodil inhibited brain-type GIRK1/2 and GIRK2 channels and cardiac-type GIRK1/4 channels at nanomolar concentrations or more in a distinctive manner. The inhibition of GIRK channels by ifenprodil was concentration-dependent, but voltage-independent and time-independent with a primarily significant effect on the instantaneous current and a steady percentage inhibition during each voltage pulse. Our results also suggest that ifenprodil acted at the channels from the extracellular side of the cell membrane. On the other hand, blockade by extracellular Ba2+ and Cs+, typical of Kir channel blockers that occlude the pore of the open channel, shows a concentration-dependence, a strong voltage-dependence, and a time-dependence with a comparatively small effect on the instantaneous current but a marked inhibition on the steady-state current at the end of voltage pulses (Lesage et al, 1995). These observations suggest that ifenprodil probably causes a conformational change in the GIRK channels, but does not act as an open channel blocker of the channels, as Ba2+ and Cs+ do. The action mechanism may also be involved in the incomplete blockade of GIRK currents by ifenprodil. In the present study, ifenprodil similarly inhibited GIRK currents induced by basally free G-protein βγ subunits present in oocytes, by G-proteins mediated by κOR activation, or by ethanol. Further studies using single channel experiments may be useful for understanding the mechanism of the action of ifenprodil on GIRK channels. In addition, the potency of inhibition by Ifenprodil of GIRK1/4 channels was higher than that of GIRK1/2 and GIRK2 channels. Although the rank order of the effectiveness by ifenprodil at the highest concentrations tested was GIRK2>GIRK1/2⩾GIRK1/4 channels, the differences were not statistically significant. Moreover, Kir1.1 and Kir2.1 channels in other Kir channel subfamilies were insensitive to ifenprodil. Further studies using GIRK/Kir1.1 and GIRK/Kir2.1 chimeric channels and mutant GIRK channels may clarify the critical sites mediating the effects of ifenprodil on GIRK channels. Furthermore, high-resolution structure analysis of GIRK channels may allow characterization of the binding sites. Additionally, although haloperidol is structurally related to ifenprodil (Williams, 2001), haloperidol weakly inhibits GIRK1/2 and GIRK1/4 channels in a similar manner (Kobayashi et al, 2000). The different effectiveness of these drugs on GIRK channels may be due to the different chemical structures between them or to their different binding sites on GIRK channels. Studies on the relationship between the structures of GIRK channels and the structure of ifenprodil may provide the basis for designing candidates for potent GIRK inhibitors. Clinical and Pharmacological Implications [2] The human plasma concentrations of Ifenprodil are reported to be approximately 0.1 μM. after a single administration of its clinical dosage (Aventis Pharma's data). In animals, the radioactive ifenprodil in the brain and heart after its intramuscular administration was approximately 5–8 times and 5–10 times higher, respectively, than that in blood (Nakagawa et al, 1975). Therefore, the present findings suggest that GIRK channels in the brain and heart may be inhibited by ifenprodil at clinically relevant concentrations in these tissues. Activation of GIRK channels in physiological conditions induces K+ efflux, leading to membrane hyperpolarization (North, 1989), whereas inhibition of GIRK channels leads to a depolarization of the membrane potential, resulting in an increase in cell excitability (Kuzhikandathil and Oxford, 2002). Therefore, in clinical use ifenprodil might affect various brain and heart functions via the inhibition of GIRK channels, which are expressed widely in the nervous system and the atrium (Kobayashi et al, 1995; Karschin et al, 1996). GIRK2 knockout mice show spontaneous seizures and are more susceptible to seizures induced by pentylenetetrazol, a GABAA receptor antagonist, than wild-type mice (Signorini et al, 1997). In addition, the resting membrane potentials of neurons in GIRK knockout mice were depolarized compared to those in wild-type mice (Lüscher et al, 1997; Torrecilla et al, 2002). High doses of Ifenprodil potentiated seizures induced by some convulsants including pentylenetetrazol (Mizusawa et al, 1976), although ifenprodil has been shown to have anticonvulsant effects (Thurgur and Church, 1998; Yourick et al, 1999), probably due to inhibition of NMDA receptor channels (Williams, 2001) and Ca2+ channels (Church et al, 1994; Bath et al, 1996). In spite of its anticonvulsant property, potent blockade of neuronal GIRK channels by ifenprodil may contribute to the increased susceptibility to seizure by causing an increase in neuronal excitability. Interestingly, GIRK2 knockout mice show reduced anxiety with an increase in motor activity in three tests for anxiety: the elevated plus-maze, light/dark box, and canopy test (Blednov et al, 2001). Ifenprodil had an anxiolytic property with an increase in locomotion in MF1 mice in the elevated plus-maze test (Fraser et al, 1996), although it had no anxiolytic effect in the light/dark exploratory test and caused no change in locomotor activity in Wistar rats (Mikolajczak et al, 2003). This discrepancy might have been caused by differences in the behavioral tests including difference in the ratio of the two light/dark compartments in the apparatus and/or in animal species. A clinical study showed that Ifenprodil improved anxiety, a decrease in spontaneity, and melancholy in patients with sequelae of cerebrovascular diseases (Otomo et al, 1976). Therefore, inhibition of neuronal GIRK channels by ifenprodil might partly contribute to the clinical effects on anxiety and decreased activity, which are observed in some neuropsychiatric disorders as well. In the heart, acetylcholine opens atrial GIRK channels via activation of the M2 muscarinic acetylcholine receptor, and ultimately causes slowing of the heart rate (Brown and Birnbaumer, 1990). Sinus tachycardia during treatment with ifenprodil is observed along with its hypotensive effect (Carron et al, 1971; Young et al, 1983; Yajima et al, 1987). Ifenprodil exhibits no significant affinity for the muscarinic acetylcholine receptor (Chenard et al, 1991). The present study demonstrated that Ifenprodil, at submicromolar concentrations or more, inhibited cardiac-type GIRK1/4 channels, which are abundantly present in the atrium of the heart (Krapivinsky et al, 1995). Therefore, atrial GIRK channels may also be inhibited by ifenprodil in clinical practice. GIRK1 or GIRK4 knockout mice show mild tachycardia (Bettahi et al, 2002). Additionally, the hypotensive effect of ifenprodil may induce compensational activation of the sympathetic nervous system, which plays an important role in the stimulatory regulation of the heart rate. Taken together, our data suggest that sinus tachycardia during treatment with ifenprodil may be partly related to inhibition of atrial GIRK channels. Ifenprodil influenced ethanol-related behavioral changes in animals, such as suppression of amnestic effects and withdrawal signs including convulsions (Malinowska et al, 1999; Napiórkowska-Pawlak et al, 2000; Narita et al, 2000). Ethanol activates GIRK channels (Kobayashi et al, 1999; Lewohl et al, 1999). The present study demonstrated that ifenprodil inhibited GIRK1/2 currents induced by ethanol. Interestingly, GIRK2 knockout mice show reduced ethanol-induced conditioned taste aversion and conditioned place preference (Hill et al, 2003), and are less sensitive to some of acute ethanol effects, including anxiolysis, habituated locomotor stimulation and handling-induced convulsions after an acute administration of ethanol, than wild-type mice (Blednov et al, 2001). Taken together, ifenprodil might suppress GIRK-related ethanol effects. Morphine, a commonly used potent analgesic, preferentially binds to the μ-opioid receptor, and exerts various pharmacological effects, including analgesia, euphoria, and dependence (Gutstein and Akil, 2001). The μ-opioid receptor is coupled to G-protein-mediated signal transductions involving GIRK channels, adenylyl cyclase, Ca2+ channels, and phospholipase C (Ikeda et al, 2002). Although morphine produces a conditioned place preference in animals, indicating its rewarding effect, pretreatment with ifenprodil suppresses the rewarding effect produced by morphine (Suzuki et al, 1999). However, Ifenprodil exhibits no significant affinity for the opioid receptors (Chenard et al, 1991). The present study demonstrated that ifenprodil inhibited G-protein-mediated GIRK currents. It may be important to determine whether GIRK channel function contributes to the rewarding effect of morphine. Interestingly, GIRK knockout mice show reduced self-administration of cocaine (Morgan et al, 2003). In a clinical report, desipramine, which acts as an inhibitor of GIRK channels as well as of norepinephrine transporters (Kobayashi et al, 2004b), facilitated initial abstinence from cocaine (Gawin et al, 1989). Thus, selective GIRK inhibitors might be potential agents for the treatment of abusers of cocaine. Further studies on the effects of ifenprodil on GIRK knockout mice might clarify the roles of the GIRK-mediated effects of ifenprodil in addiction to morphine and cocaine. [2] Decisively, the polykaryon formation assay verified that nylidrin targets the HA2-mediated membrane fusion by blocking pH-dependent conformational change of HA. As above-mentioned, additional evidence supporting this hypothesis was provided by subtype dependency on the antiviral efficacy: nylidrin, Ifenprodil, and clenbuterol were active against all A/H1N1 strains tested; in contrast, nylidrin and Ifenprodil were limitedly effective only against A/Hong Kong/8/1968 and A/Seoul/11/1988 among the H3N2 strains tested (Table 1 and Table 2). This observation is intriguing because most HA2 fusion inhibitory agents, either therapeutic antibodies or small molecules, tend to exhibit antiviral potency in a viral subtype- or HA group-dependent manner; group I consists of H1, H2, and H5, whereas group 2 consists of H3 and H7. The strain-specific activity of the compounds against the H3N2 subtype viruses indicated that optimization of nylidrin through chemical modifications could be a plausible approach for identifying a broad-spectrum fusion inhibitor.[4] Influenza A virus, one of the major human respiratory pathogens, is responsible for annual seasonal endemics and unpredictable periodic pandemics. Despite the clinical availability of vaccines and antivirals, the antigenic diversity and drug resistance of this virus makes it a persistent threat to public health, underlying the need for the development of novel antivirals. In a cell culture-based high-throughput screen, a β2-adrenergic receptor agonist, nylidrin, was identified as an antiviral compound against influenza A virus. The molecule was effective against multiple isolates of subtype H1N1, but had limited activity against subtype H3N2, depending on the strain. By examining the antiviral activity of its chemical analogues, we found that Ifenprodil and clenbuterol also had reliable inhibitory effects against A/H1N1 strains. Field-based pharmacophore modeling with comparisons of active and inactive compounds revealed the importance of positive and negative electrostatic patterns of phenyl aminoethanol derivatives. Time-of-addition experiments and visualization of the intracellular localization of nucleoprotein NP demonstrated that an early step of the virus life cycle was suppressed by nylidrin. Ultimately, we discovered that nylidrin targets hemagglutinin 2 (HA2)-mediated membrane fusion by blocking conformational change of HA at acidic pH. In a mouse model, preincubation of a mouse-adapted influenza A virus (H1N1) with nylidrin completely blocked intranasal viral infection. The present study suggests that nylidrin could provide a core chemical skeleton for the development of a direct-acting inhibitor of influenza A virus entry.[4] |
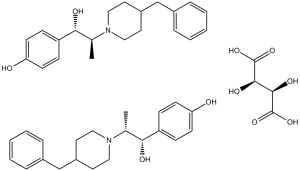
| 分子式 |
C21H27NO2.1/2C4H6O6
|
|
|---|---|---|
| 分子量 |
800.98
|
|
| 精确质量 |
325.204
|
|
| 元素分析 |
C, 68.98; H, 7.55; N, 3.50; O, 19.97
|
|
| CAS号 |
23210-58-4
|
|
| 相关CAS号 |
Ifenprodil glucuronide;66516-92-5
|
|
| PubChem CID |
3689
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 沸点 |
493.5ºC at 760mmHg
|
|
| 熔点 |
178-180ºC
|
|
| 闪点 |
248.7ºC
|
|
| 蒸汽压 |
0mmHg at 25°C
|
|
| LogP |
1.584
|
|
| tPSA |
158.76
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
24
|
|
| 分子复杂度/Complexity |
353
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
CC(C(C1=CC=C(C=C1)O)O)N2CCC(CC2)CC3=CC=CC=C3.CC(C(C1=CC=C(C=C1)O)O)N2CCC(CC2)CC3=CC=CC=C3.[C@@H]([C@H](C(=O)O)O)(C(=O)O)O
|
|
| InChi Key |
DMPRDSPPYMZQBT-CEAXSRTFSA-N
|
|
| InChi Code |
InChI=1S/2C21H27NO2.C4H6O6/c2*1-16(21(24)19-7-9-20(23)10-8-19)22-13-11-18(12-14-22)15-17-5-3-2-4-6-17;5-1(3(7)8)2(6)4(9)10/h2*2-10,16,18,21,23-24H,11-15H2,1H3;1-2,5-6H,(H,7,8)(H,9,10)/t;;1-,2-/m..1/s1
|
|
| 化学名 |
4-(2-(4-benzylpiperidin-1-yl)-1-hydroxypropyl)phenol; (2R,3R)-2,3-dihydroxysuccinate (2:1)
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1 mg/mL (2.50 mM) (饱和度未知) in 1% DMSO + 99% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 12.22 mg/mL (30.51 mM) in 0.5% CMC-Na/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.2485 mL | 6.2424 mL | 12.4847 mL | |
| 5 mM | 0.2497 mL | 1.2485 mL | 2.4969 mL | |
| 10 mM | 0.1248 mL | 0.6242 mL | 1.2485 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01896388 | Completed | Drug: Ifenprodil Tartrate Drug: Placebo |
Posttraumatic Stress Disorders | Chiba University | January 21, 2014 | Phase 1 Phase 2 |
| NCT06330077 | Not yet recruiting | Drug: Ifenprodil Drug: Placebo |
Multiple Sclerosis Remitting Relapsing Multiple Sclerosis |
Assistance Publique - Hôpitaux de Paris | March 2024 | Phase 2 |
| NCT04382924 | Completed Has Results | Drug: NP-120 (Ifenprodil) | COVID | Algernon Pharmaceuticals | August 5, 2020 | Phase 2 Phase 3 |
| NCT04318704 | Completed | Drug: Ifenprodil | Idiopathic Pulmonary Fibrosis | Algernon Pharmaceuticals | July 29, 2020 | Phase 2 |
|
|---|
|