| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Glucocorticoid Receptor (GR)[1][2][5]
|
|---|---|
| 体外研究 (In Vitro) |
洛替泼诺具有代谢不稳定的功能,即 17β-酯,旨在在体循环中快速失活。依碳氯替泼诺表现出的结合亲和力是地塞米松的 4.3 倍,这两种化合物的 Hill 因子接近 1,而 PJ90 和 PJ91 对受体没有表现出任何亲和力。激酶测定: 细胞测定:
在人肥大细胞和嗜酸性粒细胞(过敏反应中的关键炎症细胞)中,氯替泼诺依碳酸酯(10-1000 nM)以剂量依赖方式抑制IgE介导的脱颗粒。100 nM浓度时,ELISA检测显示组胺释放减少55%,白三烯C4分泌减少48%;RT-PCR检测显示促炎细胞因子(IL-4、IL-5、TNF-α)表达降低35-42%[2] - 在兔角膜上皮细胞和结膜成纤维细胞中,氯替泼诺依碳酸酯(0.1-10 μM)抑制LPS诱导的炎症。1 μM浓度时,Western blot检测显示环氧合酶-2(COX-2)和诱导型一氧化氮合酶(iNOS)蛋白表达降低40-45%,减少一氧化氮(NO)和前列腺素E2(PGE2)的产生[1][3] - 在人支气管上皮细胞中,氯替泼诺依碳酸酯(50-500 nM)在200 nM浓度时抑制TNF-α诱导的ICAM-1表达50%(免疫荧光检测),阻止白细胞与上皮细胞的黏附[2] |
| 体内研究 (In Vivo) |
静脉注射 5 mg/kg 依碳氯替泼诺的犬显示出 2.8 小时的终末半衰期、3.7 L/kg 的分布容积和 0.9 L/h/kg 的全身清除率。尿液中未发现。狗口服药物(5毫克/公斤),血浆中仅含有代谢物——没有完整的药物——这表明存在显着的首过影响。
在卵清蛋白诱导的兔过敏性结膜炎模型中,局部给予氯替泼诺依碳酸酯滴眼液(0.5%,每日4次,连续7天),较溶媒对照组减少结膜充血、水肿和瘙痒评分60-70%;组织病理学分析显示结膜组织中嗜酸性粒细胞浸润减少58%[1][3] - 在卵清蛋白致敏的小鼠过敏性哮喘模型中,吸入氯替泼诺依碳酸酯(0.1-1 mg/kg,每日1次,连续14天)剂量依赖地降低对乙酰甲胆碱的气道高反应性(1 mg/kg时降低45%),并减少支气管肺泡灌洗液(BALF)中炎症细胞计数(嗜酸性粒细胞、中性粒细胞)50-65%[2] - 在内源性前葡萄膜炎患者中,局部使用氯替泼诺依碳酸酯(0.5%滴眼液,每日4次,连续2周),前房炎症(细胞和闪辉评分)降低75%,80%的患者眼部疼痛和畏光症状缓解[4][5] |
| 酶活实验 |
糖皮质激素受体(GR)结合检测:将重组人GR配体结合域与[3H]-地塞米松(GR参考激动剂)及梯度浓度(1-100 nM)的氯替泼诺依碳酸酯在25°C孵育2小时。凝胶过滤法分离结合态配体,定量放射性强度,评估其与GR的竞争性结合能力[1][5]
|
| 细胞实验 |
肥大细胞脱颗粒实验:人肥大细胞经IgE致敏后,用10 nM、100 nM、1000 nM的氯替泼诺依碳酸酯预处理1小时,再用特异性抗原刺激。ELISA检测上清液中组胺和白三烯C4水平,RT-PCR检测促炎细胞因子mRNA表达[2]
- 角膜上皮细胞炎症实验:兔角膜上皮细胞接种到6孔板,用0.1 μM、1 μM、10 μM的氯替泼诺依碳酸酯预处理2小时,再用1 μg/mL LPS刺激24小时。Western blot分析COX-2和iNOS蛋白水平;比色法和ELISA分别定量NO和PGE2的产生[1][3] - 支气管上皮细胞ICAM-1表达实验:人支气管上皮细胞接种到96孔板,用50 nM、200 nM、500 nM的氯替泼诺依碳酸酯处理1小时,再用10 ng/mL TNF-α刺激18小时。免疫荧光检测ICAM-1表达,通过与标记的中性粒细胞共培养评估白细胞黏附情况[2] |
| 动物实验 |
Allergic conjunctivitis rabbit model: New Zealand white rabbits were sensitized with ovalbumin via intraperitoneal injection. After 2 weeks, Loteprednol etabonate eye drops (0.2%, 0.5%) were administered topically 4 times daily for 7 days, starting 1 day before ovalbumin ocular challenge. Conjunctival symptoms (redness, edema, itching) were scored daily; conjunctival tissues were collected for histopathological analysis of eosinophil infiltration[1][3]
- Allergic asthma mouse model: BALB/c mice were sensitized with ovalbumin and aluminum hydroxide, then challenged with ovalbumin aerosol. Loteprednol etabonate (0.1 mg/kg, 0.5 mg/kg, 1 mg/kg) was administered via inhalation once daily for 14 days during the challenge phase. Airway hyperresponsiveness was measured by methacholine provocation; BALF was collected to count inflammatory cells[2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Loteprednol etabonate (LE) demonstrates good ocular permeation properties as it is lipid soluble, allowing the agent to penetrate into cells with relative ease. Results from the ocular administration of loteprednol in normal, healthy volunteers have shown that there are low or undetectable concentrations of either unchanged material or its metabolite. Following twice-daily unilateral topical ocular dosing of LE for 14 days in healthy subjects, the plasma concentrations of loteprednol etabonate were below the limit of quantitation (1 ng/mL) at all time points. These finds suggest that limited, if any, systemic absorption of LE occurs. Following systemic administration to rats, loteprednol etabonate is eliminated primarily via the biliary/faecal route, with most of the dose eliminated in the form of the metabolite, PJ-90. The only data available regarding the volume of distribution of loteprednol etabonate (LE) is the volume of distribution the agent demonstrated when administered to dogs - a value of 3.7 L/kg. It has been shown, however, that the topical ocular administration of LE distributes preferentially into the cellular components of blood. Loteprednol etabonate was slowly hydrolyzed in liver at clearance rates of 0.21 +/- 0.04 and 2.41 +/- 0.13 ml/h/kg in the liver and plasma, respectively. Metabolism / Metabolites Loteprednol etabonate (LE) is readily and extensively metabolized to two inactive metabolites, PJ-90 (Δ1-cortienic acid) and PJ-91 (Δ1-cortienic acid etabonate). Metabolism occurs locally in ocular tissues, and to the extent that loteprednol etabonate reaches the systemic circulation, likely the liver and other tissues into which it distributes. In particular, studies have demonstrated that LE (chloromethyl 17alpha-ethoxycarbonyloxy-11beta-hydroxy-3-oxoandrosta-1,4-diene) is rapidly hydrolyzed at the location of its 17beta-chloromethyl ester function by paraoxonase 1 in human plasma at the site of administration at the level of the affected eye tissue to the 17beta-carboxylate PJ-91 metabolite and PJ-90 metabolite. Both metabolites are considered inactive. Biological Half-Life The terminal half-life of loteprednol etabonate as determined when administered intravenously at a dose of 5 mg/kg in the dog animal model is 2.8 hours. Absorption: Topical ocular administration (0.5% eye drops) results in minimal systemic absorption (<0.1% of dose detected in plasma). Inhaled administration shows low systemic bioavailability (~2%)[1][2] - Distribution: After topical ocular application, it primarily distributes in ocular tissues (cornea, conjunctiva, anterior chamber) with minimal penetration into the posterior segment of the eye[3][5] - Metabolism: Rapidly metabolized in the liver and target tissues via esterase hydrolysis to inactive carboxylic acid metabolites. The elimination half-life in plasma is ~1 hour[1][5] - Excretion: Metabolites are primarily excreted in urine (~70%) and feces (~25%), with no accumulation of parent drug[1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Strong protein binding of approximately 98% for loteprednol etabonate facilitates little pharmacodynamic action and/or adverse effects on the part of the agent in the systemic circulation. Local toxicity: Ocular administration shows low risk of (IOP) elevation (incidence <5%, vs. 15-20% with prednisolone). No significant corneal epithelial toxicity or conjunctival irritation in long-term use (up to 6 weeks)[3][4] - Systemic toxicity: No significant (liver/renal function markers within normal ranges) even at 10-fold therapeutic doses[1][5] - Plasma protein binding rate: ~80% bound to human plasma proteins[1] - Drug-drug interactions: No significant interactions with other ocular or systemic drugs; does not inhibit or induce cytochrome P450 enzymes[5] |
| 参考文献 |
|
| 其他信息 |
Pharmacodynamics
Loteprednol etabonate (LE) belongs to a unique class of corticosteroids with potent anti-inflammatory effects designed to be active at the site of action. Animal studies have shown that LE has a binding affinity to steroid receptors that is 4.3 times greater than dexamethasone. This particular class of steroids consists of bioactive molecules whose in-vivo transformation to non-toxic substances can be predicted from their chemistry and knowledge of enzymatic pathways in the body. Cortienic acid is an inactive metabolite of hydrocortisone and analogs of cortienic acid are also devoid of corticosteroid activity. Specifically, LE is an ester derivative of one of these analogs, cortienic acid etabonate. In particular, LE possesses a metabolically labile 17 beta-chloromethyl ester function which was designed in order to be hydrolyzed to an inactive carboxylic acid moiety. This inactive metabolite is more hydrophilic and is thus readily eliminated from the body. LE also exhibits good ocular permeation properties and good skin permeation properties. Loteprednol etabonate is a "soft steroid" (prodrug-like) developed for topical anti-inflammatory therapy, with high potency and low systemic toxicity[1][2][5] - Its core mechanism involves binding to GR, inhibiting the transcription of pro-inflammatory genes (cytokines, chemokines, COX-2) and activating anti-inflammatory gene expression[1][5] - Clinical indications include ocular inflammatory conditions (allergic conjunctivitis, anterior uveitis, blepharitis) and airway allergic diseases (allergic rhinitis, mild-to-moderate asthma)[2][4] - It is FDA-approved for ocular use and has a favorable safety profile compared to conventional corticosteroids, due to rapid metabolism to inactive metabolites[3][5] - The ester linkage in its structure is critical for local activity and rapid inactivation, minimizing systemic side effects[1][2] |
| 分子式 |
C24H31CLO7
|
|
|---|---|---|
| 分子量 |
466.95
|
|
| 精确质量 |
466.175
|
|
| CAS号 |
82034-46-6
|
|
| 相关CAS号 |
Loteprednol Etabonate-d5;2026643-11-6;Loteprednol Etabonate-d3
|
|
| PubChem CID |
444025
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
600.1±55.0 °C at 760 mmHg
|
|
| 熔点 |
220.5-223.5ºC
|
|
| 闪点 |
316.7±31.5 °C
|
|
| 蒸汽压 |
0.0±3.9 mmHg at 25°C
|
|
| 折射率 |
1.571
|
|
| LogP |
3.17
|
|
| tPSA |
99.13
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
7
|
|
| 重原子数目 |
32
|
|
| 分子复杂度/Complexity |
882
|
|
| 定义原子立体中心数目 |
7
|
|
| SMILES |
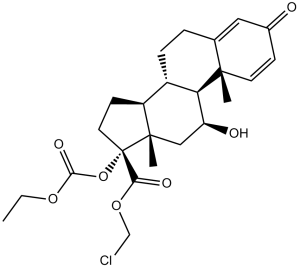
CCOC(=O)O[C@@]1(CC[C@@H]2[C@@]1(C[C@@H]([C@H]3[C@H]2CCC4=CC(=O)C=C[C@]34C)O)C)C(=O)OCCl
|
|
| InChi Key |
DMKSVUSAATWOCU-HROMYWEYSA-N
|
|
| InChi Code |
InChI=1S/C24H31ClO7/c1-4-30-21(29)32-24(20(28)31-13-25)10-8-17-16-6-5-14-11-15(26)7-9-22(14,2)19(16)18(27)12-23(17,24)3/h7,9,11,16-19,27H,4-6,8,10,12-13H2,1-3H3/t16-,17-,18-,19+,22-,23-,24-/m0/s1
|
|
| 化学名 |
chloromethyl (8S,9S,10R,11S,13S,14S,17R)-17-ethoxycarbonyloxy-11-hydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthrene-17-carboxylate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.35 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1416 mL | 10.7078 mL | 21.4156 mL | |
| 5 mM | 0.4283 mL | 2.1416 mL | 4.2831 mL | |
| 10 mM | 0.2142 mL | 1.0708 mL | 2.1416 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。