| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
p70S6K (IC50 = 4 nM)
|
|---|---|
| 体外研究 (In Vitro) |
LY2584702 抑制 HCT116 结肠癌细胞中 S6 核糖体蛋白 (pS6) 的磷酸化,IC50 为 0.1-0.24 μM。 [1] LY2584702与mTOR抑制剂依维莫司或EGFR抑制剂厄洛替尼联合使用时,表现出显着的协同效应。 [2]
RPS6KB1抑制剂LY2584702显著降低了非小细胞肺癌细胞系中RPS6KB1-rpS6的磷酸化[4] LY2584702用于抑制肺腺癌细胞系A549和鳞状细胞癌细胞系SK-MES-1中RPS6KB1的磷酸化。正如预期的那样,处理24小时后,即使在0.1μM的浓度下,A549中RPS6KB1的磷酸化也显著降低(图4,P<0.001);而SK-MES-1中p-RPS6KB1的表达似乎在0.2μM时开始下降,并随着药物浓度的增加而持续下调(图4,所有p<0.05)。RPS6KB1的公认靶点rpS6的磷酸化也同步下降(图4,所有P<0.05)。然而,无论药物浓度如何,RPS6KB1和S6的总蛋白水平都没有显著差异(图4,所有P>0.05) 0.1μM的LY2584702处理24小时后,A549的增殖受到显著抑制(图5A,P<0.05);随着治疗时间的延长和/或药物浓度的增加,下降趋势更加明显(图5A,均P<0.05)。在SK-MES-1中也观察到了类似的结果,尽管LY2584702在0.6μM时产生了明显的抑制作用(图5B,所有P<0.05),远高于A549。 基于上述结果,收集经0.2μM LY2584702处理72小时的A549进行细胞周期测定和凋亡分析。将仅用培养基或添加DMSO的培养基培养72小时的A549细胞系用作对照。毫不奇怪,更多经LY2584702处理的细胞被阻滞在G0-G1期(图6A,两者均P<0.05);S期或G2-M期细胞相应减少(图6A,均P<0.05)。此外,通过Annexin V-APC/7-AAD凋亡检测,LY2584702诱导了更多的A549细胞凋亡(图7A,均P<0.05) 由于SK-MES-1对LY2584702的敏感性较低,因此采用1μM处理72小时的SK-MES.1进行细胞周期和凋亡分析。与对照组相比,LY2584702治疗还导致SK-MES-1 G0-G1期阻滞,S期和G2-M期同步减少(图6B,所有P<0.05)。然而,LY2584702对SK-MES-1细胞凋亡的影响有限,尽管有模糊的增加趋势(图7B,两者均P>0.05)。 |
| 体内研究 (In Vivo) |
在 U87MG 胶质母细胞瘤和 HCT116 结肠癌异种移植模型中,LY2584702(12.5 mg/kg BID)均表现出显着的抗肿瘤功效。 [1]
34名患者参加了这项I期研究,并按照QD(每日一次)或BID(每日两次)的给药方案接受LY2584702治疗。A部分剂量递增(n=22)从300mg BID开始(n=2)。由于毒性,这被缩减为每天一次25mg(n=3)、50mg(n=8)、100mg(n=3,n=6)和200mg(n=6)的剂量。B部分剂量递增(n=12)包括50 mg(n=3)、75 mg(n=3)和100 mg(n=6)BID。7名患者出现了剂量限制性毒性(DLT)。所有DLT均为3级,包括呕吐、脂肪酶升高、恶心、低磷血症、疲劳和胰腺炎。 结论:MTD确定为75mg BID或100mg QD。在这些水平上没有观察到任何反应。药代动力学分析揭示了暴露的显著差异,并确定LY2584702治疗与剂量增加不成比例。[1] 共招募了29名患者,其中17名在A组,12名在B组。在A组的4名患者中观察到第1周期的剂量限制性毒性(DLTs),包括3级呕吐、低磷血症、肺栓塞和凝血因子V降低。在第1周期,B组没有观察到DLTs,与研究药物相关的最常见的治疗不良事件是:疲劳、厌食、腹泻、恶心和呕吐。7名患者接受了≥4个周期的治疗(A组3例,B组4例)。最佳的总体反应是病情稳定。LY2584702的暴露累积发生在BID(每日两次)给药时。当厄洛替尼与LY2584702联合给药时,其暴露量增加。 结论:LY2584702与厄洛替尼联合用药时耐受性不佳,因此这种联合用药是不可行的。与依维莫司联合使用耐受性更好,但临床效益非常有限[2]。 |
| 酶活实验 |
RPS6KB1是核糖体蛋白S6的激酶,其分子量为70kDa,是蛋白质翻译所必需的。尽管RPS6KB1的异常激活已在多种疾病中发现,但其在非小细胞肺癌癌症中的作用和临床意义尚未得到充分研究。在这项研究中,我们发现RPS6KB1在非小细胞肺癌中过磷酸化(p-RPM6KB1),这是一个独立的不良预后标志物。尽管免疫组织化学染色(IHC)显示,非小细胞肺癌标本中总RPS6KB1和p-RPS6KB2的表达频繁,但只有p-RPS6KB1与非小细胞癌受试者的临床病理特征相关。Kaplan-Meier生存分析显示,p-RPS6KB1表达的增加表明非小细胞肺癌患者的5年总生存率(OS)较低,而RPM6KB1阳性或阴性组之间的差异并不显著。然后使用单变量和多变量Cox回归分析来确认p-RPS6KB1的独立预后价值。为了说明非小细胞肺癌中RPS6KB1磷酸化的潜在机制,我们使用LY2584702来抑制肺腺癌细胞系A549和鳞状细胞癌细胞系SK-MES-1中RPS6KB1的磷酸化。正如预期的那样,RPS6KB1去磷酸化在CCK-8试验中显著抑制了细胞增殖,并通过细胞周期分析促进了更多细胞停滞在G0-G1期。此外,RPS6KB1去磷酸化的凋亡A549细胞急剧增加,SK-MES-1呈上升趋势,表明RPS6KBl磷酸化可能参与诱导凋亡。总之,我们的数据表明,在非小细胞肺癌中,RPS6KB1与p-RPM6KB1一样被过度激活,而不仅仅是总蛋白的过度表达。RPS6KB1的磷酸化水平可作为NSCLC患者的一种新的预后标志[4]。
|
| 细胞实验 |
LY-2584702完全溶解在20 mL 10% DMSO中并储存在-80°C。在体外进行实验时,LY-2584702在0.5% Tween 80、5%丙二醇和30% PEG400中进一步稀释,以达到0.1 μM、0.2 μM、0.6 μM和1.0 μM的各种DMSO浓度。使用细胞计数试剂盒-8 (CCK-8) 评估体外细胞增殖。将已暴露于不同浓度的 LY-2584702 24 小时的 A549 和 SK-MES-1 细胞系以每孔 5 103 个细胞的密度接种在 96 孔板中,重复六次。 LY-2584702 浓度为零时用作阴性对照,或用 DMSO 处理。接种后每 24 小时测量细胞在 450 nm 处的吸光度,以测定其增殖活性。
|
| 动物实验 |
Mice; LY-2584702 is prepared in 0.25% Tween-80 and 0.05% antifoam, and administered orally to mice (12.5 mg/kg twice daily). Injections of EOMA cells (0.3×106) are made subcutaneously into nu/nu female mice aged 6 to 8 weeks (2 sites/mouse, 4-5 mice/group). Every day, the tumor's size is determined. Animals are either given a vehicle control or the drug LY-2584702 (12.5 mg/kg twice daily, oral dosing) for treatment when tumors grow to a size of 0.01 cm3. Every 3–4 days, tumor size is determined.[3]
|
| 药代性质 (ADME/PK) |
Pharmacokinetics [1]
PK analyses were performed for both Parts A and B and, data are summarised in Table 4. The original protocol began dosing at 300 mg BID, but the two patients taking 300 mg BID experienced severe nausea and vomiting. Analysis of LY2584702 exposure level in the plasma indicated that we had exceeded the range predicted for effective target inhibition. Additionally, metabolic clearance was 10 L/h as compared to the predicted 26 L/h. Therefore, a new QD dosing scheme was implemented and ranged from 25 to 200 mg. Upon analysis of the exposures achieved with QD dosing, a BID cohort was opened to determine if BID dosing would improve total daily exposure. In the BID cohort, the MTD was determined to be 75 mg. The MTD for QD dosing was 100 mg. The half-life was conserved among cohorts at 5.96 h, but exposure (AUC) and Cmax were variable. Exposure of LY2584702 was not dose proportional but did increase with dose. LY2584702 exposures did not accumulate with QD dosing with a median accumulation ratio [AUC(0–24) day 8/AUC(0–24) day 1] of 0.61 (range: 0.52–1.7). There was accumulation with BID dosing with a median accumulation ratio of 1.98 (range: 1.1–2.69), but there was no evidence that exposure was time-dependent with median time dependency (AUC(0–24)/AUC(0–∞)) of 0.45 (range, 0.41–1.03) for QD dosing and 1.12 (range, 0.61–1.34) for BID dosing. Pharmacokinetics [2] PK parameters for LY2584702 were analysed and are summarised in (Table 4). LY2584702 exposure increased dose-proportionally when concomitantly administered with erlotinib, and the dose-normalised AUC for 50 mg QD and 50 mg BID were 88.73 ng h/mL/mg and 86.16 ng h/mL/mg, respectively. The AUC increased slightly at 75 mg BID (107.89 ng h/mL/mg) but decreased at 100 mg BID (86.11 ng h/mL/mg). The LY2584702 exposure increased dose-proportionally when administered concomitantly with everolimus. The dose-normalised AUC values were 121.95, 114.00 and 114.10 ng h/mL/mg for 50 mg BID, 50 mg QD and 100 QD, respectively. For dosing with erlotinib and everolimus, the median accumulation ratio for QD dosing was 1.09 and 1.07 (range, 0.98–1.16) and 2.16 and 1.98 (range, 1.85–3.41) for BID dosing. There was a significant difference (p = 0.0145, t-test) in LY2584702 exposure between QD and BID dosing with accumulation ratios of 1.08 and 2.46, respectively. Over all the dose groups, V/F exhibited high variability 34.52 L (47% CV). |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity [1]
Thirty-four patients received at least one dose of LY2584702, and 13 (38%) experienced a serious adverse event (SAE) during treatment. Of the 13 patients with SAE, three were related to study drug. One patient experienced Grade 3 hypophosphataemia, a second experienced Grade 3 vomiting and Grade 3 pancreatitis and a third experienced Grade 3 pancreatitis. Three patients (9%) discontinued treatment due to AE. One patient died due to progressive disease during the 30-day follow-up period after discontinuing study drug (per physician decision). This patient received one dose of study drug at 100 mg BID but was discontinued from the study due to progressive disease prior to death. Five patients in Part A and two patients in Part B experienced DLTs. All DLTs were Grade 3 and included vomiting, lipase, nausea, hypophosphataemia, fatigue and pancreatitis (Table 2). Thirty-one of 34 patients reported at least one treatment-emergent adverse event (TEAE) with 21 (62%) reporting at least one TEAE possibly related to study drug. The most common TEAEs possibly related to study drug were nausea (26%), fatigue (18%) and vomiting (15%) (Table 3). Twenty-two of 55 study drug-related AEs were ⩾Grade 3. Toxicity [2] Twenty-nine patients enrolled, and four had DLTs ⩾Grade 3: one case each of hypophosphataemia, vomiting, thromboembolic event and decreased levels of coagulation factor V (Table 2). In Arm A, eight patients (47%) experienced serious adverse events (SAEs), and six patients (50%) experienced SAEs in Arm B. SAE possibly related to study drug included: Grade 3 nausea, Grade 3 vomiting, Grade 3 anorexia, Grade 3 gastritis, grade 3 pulmonary embolism, Grade 3 increased international normalised ratio (INR), Grade 2 interstitial lung disease, Grade 2 deep vein thrombosis. Three patients (17.6%, n = 17) in Arm A and two patients (16.7%, n = 12) in Arm B discontinued treatment due to AEs. The most common drug related treatment-emergent adverse events (TEAE) were fatigue (88%), anorexia (71%), diarrhoea (65%), nausea (53%), rash acneiform (53%) and vomiting (41%) in Arm A; and fatigue (83%), anorexia (67%), nausea (58%), diarrhoea (50%) and oral mucositis (50%) in Arm B (Table 3). Eleven patients in Arm A (n = 17) and eight patients in Arm B (n = 12) experienced Grade 3/4 TEAE. Three patients in Arm A and one patient in Arm B exhibited coagulation abnormalities. One patient in Arm A experienced thromboembolic event (pulmonary embolism), and one patient in Arm B experienced a possibly related SAE of deep venous thrombosis. A third patient experienced decreased coagulation Factor V, which changed from 60% (day 1) to 24% (day 8) then recovered to 85% (day 15) and again declined to 35% (day 22) all in cycle 1. There were no clinically significant changes in intrinsic coagulation pathway factors IX, XI, XII and thromboplastin (TP). A fourth patient in Arm A experienced DLT of hypophosphataemia accompanied with increased INR along with decreases in clotting factors II, V, VII and X (Fig. 1). The changes observed were induced by treatment and improved after treatment discontinuation. Several patients in both arms experienced weight loss while on treatment and ranged from 3% to 10% in Arm A and 4–11% in Arm B. Three patients (18%) in Arm A and six patients (50%) in Arm B experienced weight loss ⩾10% of baseline (Fig. 2). Dose escalation stopped in arm B after occurrence of thromboembolic events in Arm A and B and observance of other toxicities (fatigue and weight loss). |
| 参考文献 | |
| 其他信息 |
Background: LY2584702 tosylate (hereafter referred to as LY2584702) is a potent, highly selective adenosine triphosphate (ATP) competitive inhibitor against p70 S6 kinase, a downstream component of the phosphatidylinositol-3-kinase signalling pathway which regulates cell proliferation and survival. LY2584702 exhibited anti-tumour activity in preclinical analysis.[1]
Methods: Patients with advanced solid tumours were treated with LY2584702 orally on a 28-day cycle until the criteria for maximum tolerated dose (MTD) were met. Skin biopsies were collected for pharmacodynamic analysis, and levels of phospho-S6 protein were examined. The primary objective was to determine a phase II dose and schedule with secondary objectives of observing safety and tolerability. Dose escalation was based upon Common Terminology Criteria for Adverse Events Version 3.0.[1] Results: Thirty-four patients were enrolled onto this phase I study and treated with LY2584702 on a QD (once-daily) or BID (twice-daily) dosing schedule. Part A dose escalation (n=22) began with 300 mg BID (n=2). Due to toxicity, this was scaled back to doses of 25mg (n=3), 50 mg (n=8), 100mg (n=3), and 200 mg (n=6) QD. Part B dose escalation (n=12) included 50 mg (n=3), 75 mg (n=3), and 100 mg (n=6) BID. Seven patients experienced dose-limiting toxicity (DLT). All DLTs were Grade 3 and included vomiting, increased lipase, nausea, hypophosphataemia, fatigue and pancreatitis.[1] Conclusion: The MTD was determined to be 75 mg BID or 100mg QD. No responses were observed at these levels. Pharmacokinetic analysis revealed substantial variability in exposure and determined that LY2584702 treatment was not dose proportional with increasing dose. Trial registration: ClinicalTrials.gov NCT01394003.[1] Background: LY2584702 tosylate (hereafter referred to as LY2584702) is an oral, selective ATP competitive inhibitor of p70 S6 kinase. Preclinical studies with LY2584702 demonstrated significant synergistic activity with erlotinib and everolimus. The primary objective was to determine a phase II dose and schedule. Secondary objectives included evaluation of safety, toxicity and pharmacokinetics of LY2584702 in combination with erlotinib or everolimus.[2] Methods: Patients with advanced solid tumours were treated with a total daily dose of 50-200mg of LY2584702 in combination with erlotinib 150 mg once daily (Arm A) or everolimus 10mg once daily (Arm B). Dose escalation was based on 3+3 design and used the Common Terminology Criteria for Adverse Events Version 4.0.[2] Results: Twenty-nine patients were enrolled, 17 in Arm A and 12 in Arm B. Dose limiting toxicities (DLTs) in cycle 1 were observed in Arm A in four patients and consisted of Grade 3 vomiting, hypophosphataemia, pulmonary embolism and decreased clotting factor V. No DLTs were observed in Arm B at cycle 1, and the most frequent treatment-emergent adverse events related to study drug were: fatigue, anorexia, diarrhoea, nausea and vomiting. Seven patients received ≥4 cycles (3 in A, 4 in B). Best overall response was stable disease. Exposure accumulation of LY2584702 occurred with BID (twice daily) dosing. Exposure of erlotinib increased when administered in combination with LY2584702.[2] Conclusion: LY2584702 was not well tolerated when administered with erlotinib, therefore this combination is not feasible. The combination with everolimus was better tolerated but yielded very limited clinical benefit.[2] Trial registration: ClinicalTrials.gov NCT01115803.[2] Vascular tumors are endothelial cell neoplasms whose mechanisms of tumorigenesis are poorly understood. Moreover, current therapies, particularly those for malignant lesions, have little beneficial effect on clinical outcomes. In this study, we show that endothelial activation of the Akt1 kinase is sufficient to drive de novo tumor formation. Mechanistic investigations uncovered opposing functions for different Akt isoforms in this regulation, where Akt1 promotes and Akt3 inhibits vascular tumor growth. Akt3 exerted negative effects on tumor endothelial cell growth and migration by inhibiting activation of the translation regulatory kinase S6-Kinase (S6K) through modulation of Rictor expression. S6K in turn acted through a negative feedback loop to restrain Akt3 expression. Conversely, S6K signaling was increased in vascular tumor cells where Akt3 was silenced, and the growth of these tumor cells was inhibited by a novel S6K inhibitor. Overall, our findings offer a preclinical proof of concept for the therapeutic utility of treating vascular tumors, such as angiosarcomas, with S6K inhibitors.[3] |
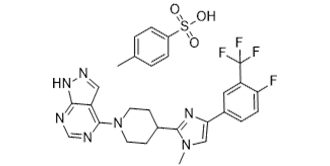
| 分子式 |
C28H27F4N7O3S
|
|---|---|
| 分子量 |
617.62
|
| 精确质量 |
617.183
|
| 元素分析 |
C, 54.45; H, 4.41; F, 12.30; N, 15.88; O, 7.77; S, 5.19
|
| CAS号 |
1082949-68-5
|
| 相关CAS号 |
LY-2584702 free base;1082949-67-4;LY-2584702 hydrochloride;1082948-81-9
|
| PubChem CID |
46205871
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
6.682
|
| tPSA |
138.27
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
12
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
43
|
| 分子复杂度/Complexity |
851
|
| 定义原子立体中心数目 |
0
|
| SMILES |
S(C1C([H])=C([H])C(C([H])([H])[H])=C([H])C=1[H])(=O)(=O)O[H].FC1C([H])=C([H])C(=C([H])C=1C(F)(F)F)C1=C([H])N(C([H])([H])[H])C(C2([H])C([H])([H])C([H])([H])N(C3C4C([H])=NN([H])C=4N=C([H])N=3)C([H])([H])C2([H])[H])=N1
|
| InChi Key |
HDYUXDNMHBQKAU-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C21H19F4N7.C7H8O3S/c1-31-10-17(13-2-3-16(22)15(8-13)21(23,24)25)29-19(31)12-4-6-32(7-5-12)20-14-9-28-30-18(14)26-11-27-20;1-6-2-4-7(5-3-6)11(8,9)10/h2-3,8-12H,4-7H2,1H3,(H,26,27,28,30);2-5H,1H3,(H,8,9,10)
|
| 化学名 |
4-[4-[4-[4-fluoro-3-(trifluoromethyl)phenyl]-1-methylimidazol-2-yl]piperidin-1-yl]-1H-pyrazolo[3,4-d]pyrimidine;4-methylbenzenesulfonic acid
|
| 别名 |
LYS6K2 tosylate; LY2584702; LY 2584702; 1082949-68-5; LY2584702 tosylate; LY-2584702 (tosylate salt); LY-2584702 tosylate salt; LY 2584702 tosylate; 4-(4-(4-(4-fluoro-3-(trifluoromethyl)phenyl)-1-methyl-1H-imidazol-2-yl)piperidin-1-yl)-1H-pyrazolo[3,4-d]pyrimidine 4-methylbenzenesulfonate; 4-[4-[4-[4-fluoro-3-(trifluoromethyl)phenyl]-1-methylimidazol-2-yl]piperidin-1-yl]-1H-pyrazolo[3,4-d]pyrimidine;4-methylbenzenesulfonic acid; 4-{4-[4-(4-fluoro-3-trifluoromethyl-phenyl)-1-methyl-1H-imidazol-2-yl]-piperidin-1-yl}-1H-pyrazolo[3,4-d]pyrimidine p-toluenesulfonate; LY-2584702; LYS-6K2; LYS 6K2; LY2584702 tosylate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~7 mg/mL (~11.3 mM)
Water: <1 mg/mL Ethanol: <1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1 mg/mL (1.62 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 10.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 1 mg/mL (1.62 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 10.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1 mg/mL (1.62 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6191 mL | 8.0956 mL | 16.1912 mL | |
| 5 mM | 0.3238 mL | 1.6191 mL | 3.2382 mL | |
| 10 mM | 0.1619 mL | 0.8096 mL | 1.6191 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Protein expression of RPS6KB1, p-RPS6KB1, rpS6 and p-rpS6 after the treatment by various LY2584702 concentrations for 24 h.PLoS One.2017 Aug 9;12(8):e0182891. |
Proliferation alteration of NSCLC cell lines with RPS6KB1 dephosphorylation by LY2584702 (CCK-8 analysis).PLoS One.2017 Aug 9;12(8):e0182891. |
Cell cycle distribution of NSCLC cell lines with RPS6KB1 dephosphorylation by LY2584702.PLoS One.2017 Aug 9;12(8):e0182891. |