| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 25g |
|
||
| 50g |
|
||
| Other Sizes |
|

| 靶点 |
AMPK; Autophagy; Mitophagy
|
|---|---|
| 体外研究 (In Vitro) |
盐酸二甲双胍(1,1-二甲基双胍盐酸盐)以浓度依赖性方式抑制ESC的增殖。对于 A-ESC,IC50 为 2.45 mM,对于 N-ESC,IC50 为 7.87 mM。与增殖期细胞相比,二甲双胍对分泌期A-ESCs中AMPK信号活性的影响更为明显[2]。在培养的大鼠肝细胞中,盐酸二甲双胍(0-500 μM)以剂量依赖性方式减少糖原产生,IC50值为196.5 μM[3]。盐酸二甲双胍的 IC50 为 5 mM,对 PC-3 细胞具有细胞毒性和活细胞作用[4]。
二甲双胍抑制 Bcl-2 和 Bcl-xl,上调 BAX 激活,促进 BIM、BAD 和 PUMA,并诱导细胞色素 c 从线粒体释放到细胞质中,直接诱导 caspase-9 介导的线粒体凋亡。 [4] 二甲双胍激活肝细胞中的AMPK;结果,乙酰辅酶a羧化酶(ACC)活性降低,脂肪酸氧化被诱导,脂肪生成酶的表达被抑制。二甲双胍或腺苷类似物激活AMPK会抑制SREBP-1的表达,SREBP-1是一种关键的脂肪生成转录因子。在二甲双胍治疗的大鼠中,SREBP-1(和其他脂肪生成)mRNA和蛋白质的肝脏表达降低;AMPK靶标ACC的活性也降低了。使用一种新型AMPK抑制剂,我们发现二甲双胍对肝细胞葡萄糖产生的抑制作用需要AMPK激活。在离体大鼠骨骼肌中,二甲双胍刺激葡萄糖摄取,同时AMPK激活。AMPK的激活为该药物的多效有益作用提供了统一的解释;这些结果还表明,调节AMPK的替代方法可用于治疗代谢性疾病。[1] 二甲双胍被广泛推荐用于治疗2型糖尿病,通过5'-AMP活化蛋白激酶(AMPK)发挥其多效性作用;然而,它对线粒体吞噬的影响仍然难以捉摸。最近的证据表明,外周血单核细胞(PBMCs)表达胰岛素受体和人类有机阳离子转运蛋白,它们被广泛用作检测2型糖尿病线粒体功能的替代品。二甲双胍治疗增加了酸性囊泡和丝裂吞噬体的形成,上调了丝裂吞噬标记物,并增强了丝裂噬通量,如LC3-II表达增加和p62蛋白水平降低所示。此外,用化合物C(一种AMPK抑制剂)预处理显著降低了二甲双胍处理细胞中自噬标志物的表达,表明二甲双胍通过AMPK途径诱导自噬。总之,二甲双胍诱导的线粒体吞噬可能通过恢复正常的线粒体表型来改善细胞功能,包括β细胞,这可能对2型糖尿病和其他线粒体相关疾病患者有益。此外,PBMCs可以用作鉴定线粒体疾病的新型诊断生物标志物。[2] 在这项研究中,我们首次揭示了二甲双胍治疗导致人肺癌细胞系A549和NCI-H1299细胞凋亡增加,并以剂量和时间依赖的方式显著抑制细胞增殖,从裸鼠A549肿瘤异种移植物获得的数据进一步证明了这一点。我们还发现,二甲双胍治疗可以激活AMP活化蛋白激酶、JNK/p38 MAPK信号通路和半胱氨酸天冬氨酸蛋白酶,并上调生长停滞和DNA损伤诱导基因153(GADD153)的表达。阻断JNK/p38 MAPK通路或敲除GADD153基因都会消除二甲双胍的凋亡诱导作用。总之,我们的数据表明,二甲双胍通过激活JNK/p38 MAPK途径和GADD153抑制肺癌细胞的生长并诱导细胞凋亡。[3] 这项研究表明,二甲双胍通过诱导凋亡减少了活化HSC的数量,但不影响肝细胞的数量。二甲双胍上调BAX激活,促进BIM、BAD和PUMA;下调Bcl-2和Bcl-xl,但不影响Mcl-1。此外,二甲双胍诱导细胞色素c从线粒体释放到细胞质中,直接触发胱天蛋白酶-9介导的线粒体凋亡。线粒体膜电位(ΔΨm)的下降和线粒体中超氧化物的沉积加速了线粒体膜完整性的破坏。此外,我们验证了二甲双胍在非酒精性脂肪性肝炎(NASH)相关肝纤维化小鼠模型中的治疗效果,其中二甲双胍改善了肝功能、NASH病变和纤维化。总之,本研究表明,二甲双胍通过诱导HSC凋亡对NASH衍生的肝纤维化具有显著的治疗价值,但不影响肝细胞的增殖[4]。 |
| 体内研究 (In Vivo) |
通过组织学分析,单独使用盐酸二甲双胍(1,1-二甲基双胍盐酸盐;100 mg/kg,口服)以及二甲双胍(25、50、100 mg/kg)与 NSC 37745 组可减轻心肌细胞坏死[1]。
二甲双胍在非酒精性脂肪性肝炎 (NASH) 相关肝纤维化小鼠模型中表现出治疗作用,从而改善肝功能、NASH 病变和纤维化。 [4] 评估二甲双胍的体内效应。[1] 为了评估上述二甲双胍的选定效应是否也在体内发生,对SD大鼠进行了研究(表1)。大鼠口服二甲双胍或赋形剂(H2O)5天。大鼠饥饿20小时,然后在最后一次给药前重新喂食2小时。最后一次给药后4小时,采集组织和血液样本进行分析(见方法)。在饥饿期间,应该很少有脂质合成。重新进食后,应显著诱导肝脏脂质合成。在再喂养条件下检查了二甲双胍的作用。除了血浆胰岛素和甘油三酯的适度降低外,β-羟基丁酸酯也有小幅但显著的增加,这表明二甲双胍治疗的大鼠肝脏脂肪酸氧化是诱导的。此外,二甲双胍治疗显著降低了SREBP-1、FAS和S14的mRNA在肝脏的表达,这与细胞中记录的效果一致(表1)。 使用抗SREBP-1抗体检测大鼠肝细胞核提取物中的成熟SREBP-1蛋白(图5b)。正如预期的那样,在饥饿动物的肝核提取物中没有检测到SREBP-1成熟形式的蛋白质。在再喂养的动物中,成熟的SREBP-1蛋白的积累与这种情况下脂质合成的增加相一致。用二甲双胍治疗可以防止这种积聚。使用AICAR(500mg/kg/天)治疗后再喂养大鼠的肝核提取物获得的其他结果也表明,SREBP-1成熟形式蛋白的存在被消除。 在离体肝脏中测量AMPK活化是困难的,因为已知短暂的缺氧会导致酶的显著活化。因此,我们使用二甲双胍治疗大鼠的肝组织来确定在几种测试的柠檬酸盐浓度下ACC活性显著降低(图6)。柠檬酸盐浓度为1 mM时ACC活性降低最大(从54.6±11.8降至35.6±7.7 nmol/mg/min;P<0.01)。这些结果与二甲双胍在体内产生AMPK激活和ACC失活的结果一致。 二甲双胍缓解了非酒精性脂肪性肝炎(NASH)相关纤维化小鼠模型的肝脏病变[4] 建立了NASH相关纤维化小鼠模型(图7a),以探索二甲双胍是否能在体内疾病状态下保护肝脏。每隔一周和每三周分别测量每只小鼠的体重和生化指标(ALT和AST)。结果显示,与MCS饮食组或AIN93饮食组相比,HFMCD饮食组的体重没有显著增加(图S2a)。此外,与MCS饮食组或AIN93组相比,在HFMCD饮食治疗组中,二甲双胍降低了AST和ALT水平以及肝脏指数(图7b、图S2b)。总的来说,二甲双胍改善了NASH相关纤维化小鼠模型的肝功能。 从NASH纤维化模型中获得的石蜡包埋的肝组织样本用苏木精和伊红染色,以观察二甲双胍对肝损伤的影响。HFMCD组新鲜肝组织颜色偏深(图S2c)。结果显示,AIN93或MCS组的肝脏没有明显病变,也没有受到二甲双胍的影响(图S2d)。然而,在HFMCD预防和治疗组中,使用二甲双胍后,脂肪变性面积和炎性细胞数量均减少(图7c),表明NASH病变的严重程度减轻。此外,通过V-G染色观察纤维化程度。结果显示,HFMCD治疗组的胶原蛋白积累远少于HFMCD饮食盐水组(图7d)。Masson染色也观察到了相同的结果(图S2e)。此外,免疫组织化学染色显示,二甲双胍治疗组HSC切割半胱氨酸天冬氨酸蛋白酶-3的阳性率为38.7±5.2%,高于生理盐水组(7.69±0.61%)、空白对照组(0.58±0.03%)和二甲双胍治疗组(1.82±0.05%),表明二甲双胍诱导HSC凋亡(图7e,表3)。因此,二甲双胍延缓了小鼠模型的纤维化。 |
| 酶活实验 |
这项研究表明,二甲双胍通过诱导细胞凋亡减少了活化的HSC的数量,但不影响肝细胞的数量。二甲双胍通过促进BIM、BAD和PUMA上调BAX活化;下调Bcl-2和Bcl-xl,但不影响Mcl-1。此外,二甲双胍诱导细胞色素c从线粒体释放到细胞质中,直接触发胱天蛋白酶-9介导的线粒体凋亡。线粒体膜电位(ΔΨm)的下降和超氧化物在线粒体中的沉积加速了线粒体膜完整性的破坏。此外,我们验证了二甲双胍在与非酒精性脂肪性肝炎(NASH)相关的肝纤维化小鼠模型中的治疗效果,在该模型中,二甲双胍改善了肝功能、NASH病变和纤维化。总之,本研究表明,二甲双胍通过诱导HSC凋亡,对NASH衍生的肝纤维化具有显著的治疗价值,但不影响肝细胞的增殖[4]。
免疫沉淀AMPK测定。[1] 使用针对AMPKα1(NH2-DFYLATSPPDSFLDDHHLTR-OH)或AMPKα2(NH2-MDSAMHIPPGLKPH-OH)的多克隆抗体对来自AICAR或二甲双胍处理的大鼠肝细胞的10微克35%硫酸铵沉淀物(含AMPK)进行免疫沉淀,然后进行AMPK测定。 肌肉AMPK活性和葡萄糖摄取的测量。[1] 将分离的大鼠耳蜗上肌与二甲双胍(2 mM)或对照培养基一起孵育3小时,然后如上所述测量AMPKα1或AMPKα2活性。对于葡萄糖摄取,在3小时温育的最后30分钟,胰岛素(300 nM)存在。然后,如前所述,在二甲双胍和/或胰岛素存在或不存在的情况下,使用10分钟的温育来测量3-0-甲基葡萄糖摄取。 半胱天冬酶酶活性测定[4] 按照生产说明,用胱天蛋白酶活性测定试剂盒测量胱天蛋白酶1、3、8和9的酶活性。将2×105个细胞在冰上裂解15分钟,然后依次加入底物。酶活性用酶标仪(λ=405nm)测量。 |
| 细胞实验 |
将ESCs以1×103个细胞/孔的浓度接种在96孔板中。附着后,用不同剂量的二甲双胍/化合物C处理细胞0分钟、15分钟、1小时和24小时。如前所述进行MTT测定。简言之,将MTT(5 mg/mL)以10μL/孔的体积添加到96孔板中,并将板孵育4小时。通过去除含有MTT的培养基终止MTT反应,并添加每孔100μL DMSO,并在RT下在摇床上孵育10分钟,以确保晶体充分溶解。在595nm处测量吸光度值。细胞增殖(对照百分比)计算如下:吸光度(实验组)/吸光度(对照组)。细胞增殖抑制(对照的百分比)计算如下:100%−细胞增殖(对照的百分数)。每个实验重复进行六次,以评估结果的一致性[2]。
将大鼠 HSC T6、人肝细胞 L02 和大鼠肝细胞 BRL-3A 维持在 DMEM 中,在 37°C 的湿润环境中补充有 10% (v/v) FBS 和 1% (v/v) 青霉素/链霉素硫酸盐。 5% CO2 培养箱。人肝星状细胞 (HSC) LX-2 维持在 RPMI 1640 培养基中。通过孔径为 0.22 m 的微孔膜过滤器过滤二甲双胍和 AICAR 的溶液。 CCK8 测定用于测量不同浓度的二甲双胍和 AICAR 对肝星状细胞生长的影响。用不同剂量的二甲双胍和 AICAR 治疗 24 小时后,使用蛋白质印迹发现胶原蛋白和 -SMA 蛋白的表达。给予细胞10 mM二甲双胍和0.5 mM AICAR 24小时后,使用Western blot鉴定胶原蛋白和-SMA蛋白的表达。 RT-qPCR 用于检测 I 型胶原蛋白和 -SMA 的 mRNA 水平。 原代肝细胞中AMPK、ACC和脂肪酸氧化的测量。[1] 通过胶原酶消化从雄性Sprague-Dawley(SD)大鼠中分离肝细胞。对于AMPK测定,将细胞以1.5×106个细胞/孔的速度接种在含有100 U/ml青霉素、100μg/ml链霉素、10%FBS、100 nM胰岛素、100 nM-地塞米松和5μg/ml转铁蛋白的DMEM中的六孔板上,持续4小时。然后将细胞在无血清DMEM中培养16小时,然后用对照培养基、5-氨基咪唑甲酰胺核苷(AICAR)或指定浓度的二甲双胍处理1小时或7小时。在39小时的治疗中,对照组和二甲双胍(10或20μM)组的细胞在DMEM加5%FBS和100 nM胰岛素中培养,每12小时更换一次新鲜对照组和含二甲双胍的培养基(最后一次更换培养基是在收获前3小时)。处理后,细胞直接在含有洋地黄皂苷和磷酸酶抑制剂的缓冲液A中裂解,然后用35%饱和度的硫酸铵沉淀。通过测量合成肽底物SAMS(HMRSAMSGLHLVKRR)的磷酸化来测定AMPK活性。对于ACC测定,使用来自洋地黄皂苷裂解肝细胞的35%硫酸铵沉淀(每个4μg)在20mM柠檬酸盐存在下通过14CO2固定测定ACC活性,如前所述。对于脂肪酸氧化,14C-油酸盐氧化为酸溶性产物的过程与之前一样进行,但在没有白蛋白的培养基M199中进行。 |
| 动物实验 |
The animals are randomized into six groups consisting of six rats each. Rats in group 1 (control) receives a subcutaneous injection of physiological saline (0.5 mL) and are left untreated for the entire experimental period. Rats in group 2 receives an oral administration of metformin (100 mg/kg; twice daily) for 2 days and are subcutaneously injected with saline at an interval of 24 h for 2 consecutive days. Rats in group 3 (MI control) receives an oral administration of saline (twice daily) for 2 days and are sc injected with isoproterenol (100 mg/kg) daily for 2 consecutive days at an interval of 24 h. Rats in groups 4 to 6 are treated with metformin at 25, 50, and 100 mg/kg. Metformin is dissolved in saline and is gavaged at a volume of 0.25-0.5 mL twice a day at an interval of 12 h, started immediately before isoproterenol injection [1].
Oral gavage was used to administer 1 ml of Metformin (100 mg/ml) or water alone to male SD rats (300–350 g, n = 7–8). Rats were treated once (see Table 1and Figure 5b) or twice (see Figure 6) a day for 5 days. Rats were starved for 20 hours and then re-fed for 2 hours before the final dose; 4 hours after final dose, the animals were anesthetized and livers rapidly removed by freeze clamping followed by blood withdrawal. RNA was prepared from the freeze-clamped liver by Ultraspec RNA isolation reagent. Nuclear extracts were prepared from a pool of seven rat livers. Glucose levels were determined using the standard glucose oxidase assay kit; β-hydroxybutyrate concentrations were assayed by measuring the reduction of NAD to NADH with a standard assay kit. FFA levels were measured with the assay kit.Triglyceride levels were assayed with a kit. Insulin concentrations were measured with the enzyme-immunoassay kit.[1] Male C57BL/6N mice (6–8 weeks old, weight, 16–20 g) were randomly divided into three groups (n = 10 per group): (a) high-fat methionine/choline-deficient (HFMCD) diet group (the NASH-fibrosis model group); (b) methionine/choline-sufficient (MCS) diet group (the model control group); (c) AIN93 diet group (the blank control group). Each group were divided into two sub-groups (n = 5 per group): one was intraperitoneally injected with Metformin, the other with saline. Mice were housed in an environment where temperature, humidity and light were controlled (temperature 25 ± 2 °C, a 12/12 h light/dark cycle, 55 ∼ 60 % humidity). All mice got access to the diet and water, which were changed at a fixed time every afternoon. The normal control and model control mice were fed at the same time as the HFMCD group. All mice were divided into preventive group (intraperitoneal injection from the first week), therapeutic group (intraperitoneal injection from the eighth week) and saline group according to the administration time of metformin and saline. The concentration gradient of Metformin mice received ranged from 10 mg kg−1 to 200 mg kg−1 to get a proper concentration. Metformin was dissolved in saline. Mice received metformin or saline (65 mg kg−1 i.p.) administration every other day for 11 weeks or 4 weeks after 8 days’ post-acclimation (the preventative group) or 8 weeks’ HFMCD-diet-induced liver injury (the therapeutic group), respectively. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
**Regular tablet absorption** The absolute bioavailability of a metformin 500 mg tablet administered in the fasting state is about 50%-60%. Single-dose clinical studies using oral doses of metformin 500 to 1500 mg and 850 to 2550 mg show that there is a lack of dose proportionality with an increase in metformin dose, attributed to decreased absorption rather than changes in elimination. At usual clinical doses and dosing schedules of metformin, steady-state plasma concentrations of metformin are achieved within 24-48 hours and are normally measured at <1 μg/mL. **Extended-release tablet absorption** After a single oral dose of metformin extended-release, Cmax is reached with a median value of 7 hours and a range of between 4 and 8 hours. Peak plasma levels are measured to be about 20% lower compared to the same dose of regular metformin, however, the extent of absorption of both forms (as measured by area under the curve - AUC), are similar. **Effect of food** Food reduces the absorption of metformin, as demonstrated by about a 40% lower mean peak plasma concentration (Cmax), a 25% lower area under the plasma concentration versus time curve (AUC), and a 35-minute increase in time to peak plasma concentration (Tmax) after ingestion of an 850 mg tablet of metformin taken with food, compared to the same dose administered during fasting. Though the extent of metformin absorption (measured by the area under the curve - AUC) from the metformin extended-release tablet is increased by about 50% when given with food, no effect of food on Cmax and Tmax of metformin is observed. High and low-fat meals exert similar effects on the pharmacokinetics of extended-release metformin. This drug is substantially excreted by the kidney. Renal clearance of metformin is about 3.5 times higher than creatinine clearance, which shows that renal tubular secretion is the major route of metformin elimination. After oral administration, about 90% of absorbed metformin is eliminated by the kidneys within the first 24 hours post-ingestion. The apparent volume of distribution (V/F) of metformin after one oral dose of metformin 850 mg averaged at 654 ± 358 L. Renal clearance is about 3.5 times greater than creatinine clearance, which indicates that tubular secretion is the major route of metformin elimination. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours. Metformin is slowly and incompletely absorbed from the GI tract, mainly from the small intestine; absorption is complete within 6 hours. The absolute oral bioavailability of the drug under fasting conditions is reported to be approximately 50-60% with metformin hydrochloride doses of 0.5-1.5 g; binding of the drug to the intestinal wall may explain the difference between the amount of drug absorbed (as determined by the urinary and fecal excretion of unchanged drug) and the amount bioavailable in some studies. In single-dose studies with metformin hydrochloride conventional tablets doses of 0.5-1.5 g or 0.85-2.55 g, plasma metformin concentrations did not increase in proportion to increasing doses, suggesting an active saturable absorption process. Similarly, in single-dose studies with an extended-release tablet preparation (Glumetza) at doses of 0.5-2.5 g, plasma metformin concentrations did not increase in proportion to increasing doses. At steady state after administration of a metformin hydrochloride extended-release tablet preparation (Glucophage XR), the AUC and peak plasma concentrations were not dose proportional within the range of 0.5-2 g. However, limited data from studies in animals and in human intestinal cell cultures suggest that transepithelial transfer of metformin in the intestine may occur through a passive, nonsaturable mechanism, possibly involving a paracellular route. In several studies with another metformin hydrochloride extended-release tablet preparation (Fortamet) using doses of 1-2.5 g, metformin exposure was dose-related. Following oral administration of metformin hydrochloride (0.5-1.5 g) as conventional tablets in healthy individuals or in patients with type 2 diabetes mellitus, plasma concentrations decline in a triphasic manner. Following multiple-dose administration of metformin hydrochloride (500 mg twice daily for 7-14 days) as conventional tablets in a limited number of patients with type 2 diabetes mellitus, peak plasma concentrations remained unchanged, but trough drug concentrations were higher than with single-dose administration, suggesting some drug accumulation in a peripheral tissue compartment. No accumulation of metformin appears to occur following repeated oral doses of the drug as extended-release tablets. The principal plasma elimination half-life of metformin averages approximately 6.2 hours; 90% of the drug is cleared within 24 hours in patients with normal renal function. The decline in plasma metformin concentrations is slower after oral than after IV administration of the drug, indicating that elimination is absorption rate-limited. Urinary excretion data and data from whole blood indicate a slower terminal-elimination phase half-life of 8-20 hours (e.g., 17.6 hours)1 suggesting that the erythrocyte mass may be a compartment of distribution. Metformin is distributed rapidly in animals and humans into peripheral body tissues and fluids, particularly the GI tract; the drug also appears to distribute slowly into erythrocytes and into a deep tissue compartment (probably GI tissues). The highest tissue concentrations of metformin (at least 10 times the plasma concentration) occur in the GI tract (e.g., esophagus, stomach, duodenum, jejunum, ileum), with lower concentrations (twice the plasma concentration) occurring in kidney, liver, and salivary gland tissue. The drug distributes into salivary glands with a half-life of about 9 hours. Metformin concentrations in saliva are tenfold lower than those in plasma and may be responsible for the metallic taste reported in some patients receiving the drug. Any local effect of metformin on glucose absorption in the GI tract may be associated with the relatively high GI concentrations of the drug compared with those in other tissues. It is not known whether metformin crosses the blood-brain barrier or the placenta in humans or if the drug is distributed into human milk; however, in lactating rats, metformin is distributed into breast milk at levels comparable to those in plasma. Renal clearance is approximately 3.5 times greater than creatinine clearance, indicating that tubular secretion is the principal route of metformin elimination. Following a single 850-mg oral dose of metformin hydrochloride, renal clearance averaged 552, 491, or 412 mL/minute in nondiabetic adults, diabetic adults, or healthy geriatric individuals, respectively. Renal impairment results in increased peak plasma concentrations of metformin, a prolonged time to peak plasma concentration, and a decreased volume of distribution. Renal clearance is decreased in patients with renal impairment (as measured by decreases in creatinine clearance) and, apparently because of reduced renal function with age, in geriatric individuals. In geriatric individuals, decreased renal and plasma clearance of metformin also results in increased plasma concentrations of the drug; volume of distribution remains unaffected. For more Absorption, Distribution and Excretion (Complete) data for METFORMIN (12 total), please visit the HSDB record page. Metabolism / Metabolites Intravenous studies using a single dose of metformin in normal subjects show that metformin is excreted as unchanged drug in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) or biliary excretion. Metformin is not metabolized in the liver or GI tract and is not excreted in bile; no metabolites of the drug have been identified in humans. Metformin is not metabolized. Route of Elimination: Intravenous single-dose studies in normal subjects demonstrate that metformin is excreted unchanged in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) nor biliary excretion. Approximately 90% of the drug is eliminated in 24 hours in those with healthy renal function. Renal clearance of metformin is approximately 3.5 times that of creatinine clearance, indicating the tubular secretion is the primary mode of metformin elimination. Half Life: 6.2 hours. Duration of action is 8-12 hours. Biological Half-Life The plasma elimination half-life of metformin is 6.2 hours in the plasma. The elimination half-life in the blood is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution. The principal plasma elimination half-life of metformin averages approximately 6.2 hours ... . The drug distributes into salivary glands with a half-life of about 9 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Metformin is antihyperglycemic, not hypoglycemic agent. It does not cause insulin release from the pancreas and does not cause hypoglycemia, even in large doses. HUMAN EXPOSURE AND TOXICITY: Metformin is believed to work by inhibiting hepatic glucose production and increasing the sensitivity of peripheral tissue to insulin. It does not stimulate insulin secretion, which explains the absence of hypoglycemia. Metformin also has beneficial effects on the plasma lipid concentrations and promotes weight loss. Accumulation of metformin may occur in patients with renal impairment, and such accumulation rarely can result in lactic acidosis, a serious, potentially fatal metabolic disease. Lactic acidosis constitutes a medical emergency requiring immediate hospitalization and treatment; lactic acidosis is characterized by elevated blood lactate concentrations, decreased blood pH, electrolyte disturbances with an increased anion gap, and an increased lactate/pyruvate ratio. Lactic acidosis also may occur in association with a variety of pathophysiologic conditions, including diabetes mellitus, and whenever substantial tissue hypoperfusion and hypoxemia exist. Approximately 50% of cases of metformin-associated lactic acidosis have been reported to be fatal. No evidence of mutagenicity or chromosomal damage was observed in in vitro test systems, including human lymphocytes assay. ANIMAL STUDIES: No evidence of carcinogenic potential was seen in a 104-week study in male and female rats receiving metformin hydrochloride dosages up to and including 900 mg/kg daily or in a 91-week study in male and female mice receiving metformin hydrochloride at dosages up to and including 1500 mg/kg daily. Cancer preventive effect of metformin (MF) has been studied in mice, rats and hamsters. In the majority of cases metformin treatment leads to inhibition of carcinogenesis. No evidence of impaired fertility was observed in rats following administration of metformin hydrochloride dosages of 600 mg/kg daily. Reproduction studies in rats and rabbits given metformin hydrochloride dosages of 600 mg/kg daily have not revealed teratogenicity. No evidence of mutagenicity or chromosomal damage was observed in vivo in a micronucleus test in mice or in in vitro test systems, including microbial (Ames test) and mammalian (mouse lymphoma) assays. Pretreatment of rat cerebellar granule neurons with metformin greatly enhanced cell viability against glutamate-induced neurotoxicity. In aged male mice fed high-fat diet supplemented with metformin for 6 months, metformin decreased body fat composition and attenuated declines in motor function induced by a high fat diet. Performance in the Morris water maze test of hippocampal based memory function, showed that metformin prevented impairment of spatial reference memory associated with the high fat diet. ECOTOXICITY STUDIES: Adult fathead minnows (Pimephales promelas) were chronically exposed to metformin for 4 wk, at 40 ug/L. Metformin treatment induced significant up-regulation of messenger ribonucleic acid (mRNA) encoding the egg-protein vitellogenin in male fish, an indication of endocrine disruption. Metformin's mechanisms of action differ from other classes of oral antihyperglycemic agents. Metformin decreases blood glucose levels by decreasing hepatic glucose production, decreasing intestinal absorption of glucose, and improving insulin sensitivity by increasing peripheral glucose uptake and utilization. These effects are mediated by the initial activation by metformin of AMP-activated protein kinase (AMPK), a liver enzyme that plays an important role in insulin signaling, whole body energy balance, and the metabolism of glucose and fats. Activation of AMPK is required for metformin's inhibitory effect on the production of glucose by liver cells. Increased peripheral utilization of glucose may be due to improved insulin binding to insulin receptors. Metformin administration also increases AMPK activity in skeletal muscle. AMPK is known to cause GLUT4 deployment to the plasma membrane, resulting in insulin-independent glucose uptake. The rare side effect, lactic acidosis, is thought to be caused by decreased liver uptake of serum lactate, one of the substrates of gluconeogenesis. In those with healthy renal function, the slight excess is simply cleared. However, those with severe renal impairment may accumulate clinically significant serum lactic acid levels. Other conditions that may precipitate lactic acidosis include severe hepatic disease and acute/decompensated heart failure. Toxicity Data Acute oral toxicity (LD50): 350 mg/kg [Rabbit]. Interactions The clinical use of doxorubicin, which is a strong antineoplastic agent, is limited due to its cardiotoxic side effects. Metformin is a drug with antihyperglycemic effects, and it has been shown to have a cardioprotective effect on left ventricular function in experimental animal models of myocardial ischemia. The present study investigated the cardioprotective effect of metformin in rats with doxorubicin cardiotoxicity. Wistar albino rats were used in the study. Forty male, 10-week-old Wistar albino rats were randomly divided four groups. The control group rats were intraperitoneally administered saline solution twice a week, four doses in total. The doxorubicin group rats received doxorubicin (4 mg/kg, twice a week, cumulative dose: 16 mg/kg) intraperitoneally. The metformin group rats received metformin (250 mg/kg/day, every day for 14 days) via gavage. The doxorubicin + metformin group rats received doxorubicin and metformin at the same dose. Left ventricular functions were evaluated by using M-mode echocardiography one day after the last dose of doxorubicin. Heart tissue samples were histopathologically examined. Cardiomyocyte apoptosis was detected using in situ terminal deoxynucleotide transferase assay (TUNEL). Serum brain natriuretic peptide and C-type natriuretic peptide levels were measured. Catalase, superoxide dismutase, glutathione peroxidase, and tumor necrosis factor alpha levels were analyzed in the heart tissue. The assumptions of equality of variances and normal distribution were checked for all variables (Shapiro-Wilk test and Q-Q graphics). To identify intergroup differences, one-way variant analysis or the Kruskal-Wallis test was used. A p<0.05 value was accepted as statistically significant. Our results showed that doxorubicin treatment caused significant deterioration in left ventricular functions by echocardiography, histological heart tissue damage, and increase in cardiomyocyte apoptosis. Doxorubicin + metformin group showed protection in left ventricular function, elimination of histopathologic change, and reduced of cardiomyocyte apoptosis. The present study provided evidence that metformin has cardioprotective effects against doxorubicin cardiotoxicity. The link between inflammation and cancer has been confirmed by the use of anti-inflammatory therapies in cancer prevention and treatment. 5-aminosalicylic acid (5-ASA) was shown to decrease the growth and survival of colorectal cancer (CRC) cells. Studies also revealed that metformin induced apoptosis in several cancer cell lines. We investigated the combinatory effect of 5-ASA and metformin on HCT-116 and Caco-2 CRC cell lines. Apoptotic markers were determined using western blotting. Expression of pro-inflammatory cytokines was determined by RT-PCR. Inflammatory transcription factors and metastatic markers were measured by ELISA. Metformin enhanced CRC cell death induced by 5-ASA through significant increase in oxidative stress and activation of apoptotic machinery. Moreover, metformin enhanced the anti-inflammatory effect of 5-ASA by decreasing the gene expression of IL-1beta, IL-6, COX-2 and TNF-alpha and its receptors; TNF-R1 and TNF-R2. Significant inhibition of activation of NF-kappaB and STAT3 transcription factors, and their downstream targets was also observed. Metformin also enhanced the inhibitory effect of 5-ASA on MMP-2 and MMP-9 enzyme activity, indicating a decrease in metastasis. The current data demonstrate that metformin potentiates the antitumor effect of 5-ASA on CRC cells suggesting their potential use as an adjuvant treatment in CRC. Previous studies suggest that metformin may exert a protective effect on cisplatin-induced cytotoxicity in cancer cells, and this finding has led to a caution for considering metformin use in the treatment of cancer patients. However, in this paper we provide the first demonstration that metformin synergistically augments cisplatin cytotoxicity in the esophageal squamous cancer cell line, ECA109, under glucose-deprivation conditions, which may be more representative of the microenvironment within solid tumors; this effect is very different from the previously reported cytoprotective effect of metformin against cisplatin in commonly used high glucose incubation medium. The potential mechanisms underlying the synergistic effect of metformin on cisplatin-induced cytotoxicity under glucose-deprivation conditions may include enhancement of metformin-associated cytotoxicity, marked reduction in the cellular ATP levels, deregulation of the AKT and AMPK signaling pathways, and impaired DNA repair function. Concomitant treatment with the glucose-lowering drug metformin and the platelet aggregation inhibitor dipyridamole often occurs in patients with type 2 diabetes mellitus who have suffered a cerebrovascular event. The gastrointestinal uptake of metformin is mediated by the human equilibrative nucleoside transporter 4 (ENT4), which is inhibited by dipyridamole in preclinical studies. We hypothesized that dipyridamole lowers the plasma exposure to metformin. Eighteen healthy volunteers (mean age 23 years; 9 male) were randomized in an open-label crossover study. Subjects were allocated to treatment with metformin 500 mg twice daily in combination with dipyridamole slow-release 200 mg twice daily or to metformin alone for 4 days. After a washout period of 10 days, the volunteers were crossed over to the alternative treatment arm. Blood samples were collected during a 10-hr period after intake of the last metformin dose. The primary endpoint was the area under the plasma concentration-time curve (AUC0-12hr) and the maximum plasma metformin concentration (C max). In healthy subjects, dipyridamole did not significantly affect Cmax nor AUC0-12hr of metformin under steady-state conditions. Previous in vitro studies report that dipyridamole inhibits the ENT4 transporter that mediates gastrointestinal uptake of metformin. In contrast, co-administration of dipyridamole at therapeutic dosages to healthy volunteers does not have a clinically relevant effect on metformin plasma steady-state exposure. For more Interactions (Complete) data for METFORMIN (23 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse iv 180 mg/kg /Metformin hydrochloride/ LD50 Mouse subcutaneous 620 mg/kg /Metformin hydrochloride/ LD50 Rat subcutaneous 300 mg/kg /Metformin hydrochloride/ LD50 Rat oral 1 g/kg /Metformin hydrochloride/ For more Non-Human Toxicity Values (Complete) data for METFORMIN (10 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Hypoglycemic Agents Metformin hydrochloride tablets, USP are indicated as an adjunct to diet and exercise to improve glycemic control in adults and children with type 2 diabetes mellitus. /Included in US product label/ Metformin has been used in the management of metabolic and reproductive abnormalities associated with polycystic ovary syndrome. However, adequate and well-controlled clinical trials evaluating metformin therapy for polycystic ovary syndrome remain limited, particularly regarding long-term efficacy, and available data are conflicting regarding the benefits of the drug in ameliorating various manifestations of the condition. /NOT included in US product label/ Metformin is commercially available in fixed combination with glyburide or glipizide for use as an adjunct to diet and exercise to improve glycemic control in adults with diabetes mellitus; such fixed-combination preparations may be used as initial therapy in patients whose hyperglycemia cannot be controlled by diet and exercise alone, or as second-line therapy in patients who do not achieve adequate control of hyperglycemia with metformin or sulfonylurea monotherapy. A thiazolidinedione may be added to metformin in fixed combination with glyburide in patients who have inadequate glycemic control with fixed-combination therapy. For more Therapeutic Uses (Complete) data for METFORMIN (18 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ Lactic Acidosis: Lactic acidosis is a rare, but serious, metabolic complication that can occur due to metformin accumulation during treatment with metformin; when it occurs, it is fatal in approximately 50% of cases. Lactic acidosis may also occur in association with a number of pathophysiologic conditions, including diabetes mellitus, and whenever there is significant tissue hypoperfusion and hypoxemia. Lactic acidosis is characterized by elevated blood lactate levels (>5 mmol/L), decreased blood pH, electrolyte disturbances with an increased anion gap, and an increased lactate/pyruvate ratio. When metformin is implicated as the cause of lactic acidosis, metformin plasma levels > 5 ug/mL are generally found. The reported incidence of lactic acidosis in patients receiving metformin hydrochloride tablets, USP is very low (approximately 0.03 cases/1000 patient-years, with approximately 0.015 fatal cases/1000 patient-years). In more than 20,000 patient-years exposure to metformin in clinical trials, there were no reports of lactic acidosis. Reported cases have occurred primarily in diabetic patients with significant renal insufficiency, including both intrinsic renal disease and renal hypoperfusion, often in the setting of multiple concomitant medical/surgical problems and multiple concomitant medications. Patients with congestive heart failure requiring pharmacologic management, in particular those with unstable or acute congestive heart failure who are at risk of hypoperfusion and hypoxemia are at increased risk of lactic acidosis. The risk of lactic acidosis increases with the degree of renal dysfunction and the patient's age. The risk of lactic acidosis may, therefore, be significantly decreased by regular monitoring of renal function in patients taking metformin and by use of the minimum effective dose of metformin. In particular, treatment of the elderly should be accompanied by careful monitoring of renal function. Metformin hydrochloride tablets, USP treatment should not be initiated in patients = 80 years of age unless measurement of creatinine clearance demonstrates that renal function is not reduced, as these patients are more susceptible to developing lactic acidosis. In addition, metformin should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration or sepsis. Because impaired hepatic function may significantly limit the ability to clear lactate, metformin should generally be avoided in patients with clinical or laboratory evidence of hepatic disease. Patients should be cautioned against excessive alcohol intake, either acute or chronic, when taking metformin hydrochloride tablets, USP since alcohol potentiates the effects of metformin hydrochloride tablets, USP on lactate metabolism. In addition, metformin should be temporarily discontinued prior to any intravascular radiocontrast study and for any surgical procedure. The onset of lactic acidosis often is subtle, and accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence and nonspecific abdominal distress. There may be associated hypothermia, hypotension and resistant bradyarrhythmias with more marked acidosis. The patient and the patient's physician must be aware of the possible importance of such symptoms and the patient should be instructed to notify the physician immediately if they occur. Metformin hydrochloride tablets, USP should be withdrawn until the situation is clarified. Serum electrolytes, ketones, blood glucose and, if indicated, blood pH, lactate levels and even blood metformin levels may be useful. Once a patient is stabilized on any dose level of metformin, gastrointestinal symptoms, which are common during initiation of therapy, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms, could be due to lactic acidosis or other serious disease. Levels of fasting venous plasma lactate above the upper limit of normal but less than 5 mmol/L in patients taking metformin do not necessarily indicate impending lactic acidosis and may be explainable by other mechanisms, such as poorly controlled diabetes or obesity, vigorous physical activity or technical problems in sample handling. Lactic acidosis should be suspected in any diabetic patient with metabolic acidosis lacking evidence of ketoacidosis (ketonuria and ketonemia). Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a patient with lactic acidosis who is taking metformin, the drug should be discontinued immediately and general supportive measures promptly instituted. Because metformin hydrochloride tablets, USP are dialyzable (with a clearance of up to 170 mL/min under good hemodynamic conditions), prompt hemodialysis is recommended to correct the acidosis and remove the accumulated metformin. Such management often results in prompt reversal of symptoms and recovery. Accumulation of metformin may occur in patients with renal impairment, and such accumulation rarely can result in lactic acidosis, a serious, potentially fatal metabolic disease. The risk of developing lactic acidosis is much lower (e.g., 10-fold lower) with metformin than with phenformin (no longer commercially available in the US). However, lactic acidosis constitutes a medical emergency requiring immediate hospitalization and treatment; in such cases, metformin should be discontinued and general supportive therapy (e.g., volume expansion, diuresis) should be initiated immediately. Prompt hemodialysis also is recommended. Lactic acidosis is characterized by elevated blood lactate concentrations (exceeding 45 mg/dL), decreased blood pH (less than 7.35), electrolyte disturbances with an increased anion gap, and an increased lactate/pyruvate ratio. Lactic acidosis also may occur in association with a variety of pathophysiologic conditions, including diabetes mellitus, and whenever substantial tissue hypoperfusion and hypoxemia exist. Approximately 50% of cases of metformin-associated lactic acidosis have been reported to be fatal. However, it has been suggested that in such cases of lactic acidosis not accompanied by conditions predisposing to tissue anoxia (e.g., heart failure, renal or pulmonary disease), techniques for the elimination of metformin from the body may allow recovery rates exceeding 80%. Urinary tract infection has been reported in 8 or 1.1% of patients receiving metformin alone or in fixed combination with glipizide, respectively. Hypertension has been reported in 5.6 or 2.9-3.5% of patients receiving metformin alone or in fixed combination with glipizide, respectively. Musculoskeletal pain has been reported in 6.7 or 8% of patients receiving metformin alone or in fixed combination with glipizide, respectively. Severe acute hepatitis associated with marked elevations in serum hepatic aminotransferase values and cholestasis has been reported following initiation of metformin therapy in a patient receiving glipizide and enalapril. Accidental injury was reported in 7.3 or 5.6% of patients receiving metformin as an extended-release tablet preparation (Fortamet) or as conventional tablets, respectively. Pneumonitis with vasculitis has been reported rarely with concomitant metformin and oral sulfonylurea (e.g., glyburide) therapy. Upper respiratory tract infection was reported in 16.3 or 17.3% of patients receiving metformin or metformin in fixed combination with glyburide, respectively. Upper respiratory tract infection was reported in 8.5 or 8.1-9.9% of patients receiving metformin or metformin in fixed combination with glipizide, respectively, as initial therapy for type 2 diabetes mellitus. Upper respiratory tract infection was reported in 10.7 or 10.3% of patients receiving metformin or metformin in fixed combination with glipizide, respectively, as second-line therapy for type 2 diabetes mellitus. Upper respiratory tract infection was reported in 5.2 or 6.2% of patients receiving metformin or metformin combined with sitagliptin, respectively, in clinical trials. Rhinitis was reported in 4.2 or 5.6% of patients receiving metformin as an extended-release tablet preparation (Fortamet) or as conventional tablets, respectively. Infection was reported in 20.5 or 20.9% of patients receiving an extended-release tablet preparation (Fortamet) or conventional tablets, respectively. For more Drug Warnings (Complete) data for METFORMIN (23 total), please visit the HSDB record page. Pharmacodynamics **General effects** Insulin is an important hormone that regulates blood glucose levels. Type II diabetes is characterized by a decrease in sensitivity to insulin, resulting in elevations in blood glucose when the pancreas can no longer compensate. In patients diagnosed with type 2 diabetes, insulin is unable to exert adequate effects on tissues and cells (i.e. insulin resistance) and insulin deficiency may also be present. Metformin reduces hepatic production of glucose, decreases the intestinal absorption of glucose, and enhances insulin sensitivity by increasing both peripheral glucose uptake and utilization. In contrast with drugs of the sulfonylurea class, which lead to hyperinsulinemia, the secretion of insulin is unchanged with metformin use. **Effect on fasting plasma glucose (FPG) and Glycosylated hemoglobin (HbA1c)** HbA1c is an important periodic measure of glycemic control used to monitor diabetic patients. Fasting plasma glucose is also a useful and important measure of glycemic control. In a 29-week clinical trial of subjects diagnosed with type II diabetes, metformin decreased the fasting plasma glucose levels by an average of 59 mg/dL from baseline, compared to an average increase of 6.3 mg/dL from baseline in subjects taking a placebo. Glycosylated hemoglobin (HbA1c) was decreased by about 1.4% in subjects receiving metformin, and increased by 0.4% in subjects receiving placebo only. Adenomyosis is a finding that is associated with dysmenorrhea and heavy menstrual bleeding, associated with PI3K/AKT signaling overactivity. To investigate the effect of metformin on the growth of eutopic endometrial stromal cells (ESCs) from patients with adenomyosis and to explore the involvement of AMP-activated protein kinase (AMPK) and PI3K/AKT pathways. Primary cultures of human ESCs were derived from normal endometrium (normal endometrial stromal cells (N-ESCs)) and adenomyotic eutopic endometrium (adenomyotic endometrial stroma cells (A-ESCs)). Expression of AMPK was determined using immunocytochemistry and western blot analysis. 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assays were used to determine the effects of metformin and compound C on ESCs and also to detect growth and proliferation of ESCs. AMPK and PI3K/AKT signaling was determined by western blotting. A-ECSs exhibited greater AMPK expression than N-ESCs. Metformin inhibited proliferation of ESCs in a concentration-dependent manner. The IC50 was 2.45 mmol/l for A-ESCs and 7.87 mmol/l for N-ESCs. Metformin increased AMPK activation levels (p-AMPK/AMPK) by 2.0±0.3-fold in A-ESCs, 2.3-fold in A-ESCs from the secretory phase, and 1.6-fold in the proliferation phase. The average reduction ratio of 17β-estradiol on A-ESCs was 2.1±0.8-fold in proliferative phase and 2.5±0.5-fold in secretory phase relative to the equivalent groups not treated with 17β-estradiol. The inhibitory effects of metformin on AKT activation (p-AKT/AKT) were more pronounced in A-ESCs from the secretory phase (3.2-fold inhibition vs control) than in those from the proliferation phase (2.3-fold inhibition vs control). Compound C, a selective AMPK inhibitor, abolished the effects of metformin on cell growth and PI3K/AKT signaling. Metformin inhibits cell growth via AMPK activation and subsequent inhibition of PI3K/AKT signaling in A-ESCs, particularly during the secretory phase, suggesting a greater effect of metformin on A-ESCs from secretory phase.[5] Aim: Glycogen synthesis, and glucose and lactate production were examined in cultured rat hepatocytes preincubated with metformin (0-500 micro m) for 24 h. Methods: Cells incubated with[1-13C]-glucose and [1-13C]-lactate allowed us to study the effect of metformin on glucose production from glycogenolysis and gluconeogenesis in a detailed manner using NMR spectroscopy. 1H and 13C-filtered 1H-NMR spectra were recorded by using flow-injection technique. Results: Metformin decreased glycogen synthesis in a dose-dependent manner with an IC50 value of 196.5 micro m. This effect could not be reversed by the presence of the glycogen phosphorylase inhibitor DAB, suggesting that glycogenolysis was not affected. A clear correlation between glucose production and glycogen content (0.97 < R < 0.99; p < 0.001) and lactate production and glycogen content (0.97 < R < 0.99; p < 0.001) was observed. Moreover, a strong inhibition (62%, p < 0.001) of glucose produced from lactate/pyruvate (3 mm/0.3 mm) was observed in cells treated with 350 micro m metformin. Conclusion: Hepatocytes preincubated for 24 h in the presence of metformin at clinically relevant concentrations showed impaired glycogenesis as well as gluconeogenesis.[6] Background and aims: Prostate cancer is the most commonly diagnosed cancer in males in many populations. Metformin is the most widely used anti-diabetic drug in the world, and there is increasing evidence of a potential efficacy of this agent as an anti-cancer drug. Metformin inhibits the proliferation of a range of cancer cells including prostate, colon, breast, ovarian, and glioma lines. MicroRNAs (miRNAs) are a class of small, non- coding, single-stranded RNAs that downregulate gene expression. We aimed to evaluate the effects of metformin treatment on changes in miRNA expression in PC-3 cells, and possible associations with biological behaviour. Materials and methods: Average cell viability and cytotoxic effects of metformin were investigated at 24 hour intervals for three days using the xCELLigence system. The IC50 dose of metformin in the PC-3 cells was found to be 5 mM. RNA samples were used for analysis using custom multi-species microarrays containing 1209 probes covering 1221 human mature microRNAs present in miRBase 16.0 database. Results: Among the human miRNAs investigated by the arrays, 10 miRNAs were up-regulated and 12 miRNAs were down-regulated in the metformin-treated group as compared to the control group. In conclusion, expression changes in miRNAs of miR-146a, miR-100, miR-425, miR-193a-3p and, miR-106b in metformin-treated cells may be important. This study may emphasize a new role of metformin on the regulation of miRNAs in prostate cancer.[7] |
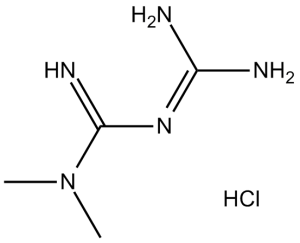
| 分子式 |
C4H11N5.HCL
|
|
|---|---|---|
| 分子量 |
165.62
|
|
| 精确质量 |
165.078
|
|
| 元素分析 |
C, 29.01; H, 7.30; Cl, 21.40; N, 42.29
|
|
| CAS号 |
1115-70-4
|
|
| 相关CAS号 |
Metformin-d6 hydrochloride;1185166-01-1; 657-24-9; 1115-70-4 (HCl); 121369-64-0 (glycinate); 58840-24-7 (orotate); 34461-22-8 ( embonate); 1384526-74-2 (icosapent)
|
|
| PubChem CID |
4091
|
|
| 外观&性状 |
White to off-white solid
|
|
| 沸点 |
224.1ºC at 760 mmHg
|
|
| 熔点 |
223-226 °C(lit.)
|
|
| 闪点 |
89.3ºC
|
|
| 蒸汽压 |
0.0929mmHg at 25°C
|
|
| LogP |
1.058
|
|
| tPSA |
88.99
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
1
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
9
|
|
| 分子复杂度/Complexity |
132
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
N(/C(=N\[H])/N=C(\N([H])[H])/N([H])[H])(C([H])([H])[H])C([H])([H])[H]
|
|
| InChi Key |
OETHQSJEHLVLGH-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C4H11N5.ClH/c1-9(2)4(7)8-3(5)6;/h1-2H3,(H5,5,6,7,8);1H
|
|
| 化学名 |
1,1-Dimethylbiguanide hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 3 mg/mL (18.11 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 3 mg/mL (18.11 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: 100 mg/mL (603.79 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.0379 mL | 30.1896 mL | 60.3792 mL | |
| 5 mM | 1.2076 mL | 6.0379 mL | 12.0758 mL | |
| 10 mM | 0.6038 mL | 3.0190 mL | 6.0379 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Voxelotor CYP and Transporter Cocktail Interaction Study
CTID: NCT05981365
Phase: Phase 1 Status: Completed
Date: 2024-11-22