| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
α2-adrenergic receptor ( pKi = 6.95 ); 5-HT3 Receptor ( pKi = 8.1 ); 5-HT2 Receptor ( pKi = 8.05 ); H1 Receptor ( pKi = 9.3 )
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:米氮平对克隆的人 α2A-肾上腺素 (AR) 受体表现出显着的亲和力,可阻断去甲肾上腺素 (NA) 诱导的鸟苷-5-O-(3-[35S]硫代)-三磷酸 ([35S] ]-GTPgammaS) 结合。米氮平对克隆的人血清素 (5-HT)2C 受体表现出高亲和力,可消除 5-HT 诱导的磷酸肌醇生成。米氮平显着提高透析液中 NA 的水平,在 FCX 中,提高 DA 的水平,而 5-HT 不受影响。米氮平通过阻断 α2 肾上腺素能自身受体和异质受体来增强 5-HT 上行通路电刺激的有效性。米氮平可阻断微离子导入去甲肾上腺素 (NE) 对 CA3 背侧海马锥体神经元放电活动的抑制作用,表明它们对突触后 α-2 肾上腺素受体具有拮抗作用。
|
| 体内研究 (In Vivo) |
Mirtazapine (10-250 mg/kg iv) 可以剂量依赖性地增强初始大鼠中 5-HT 神经元的放电活性,但在 6-羟基多巴胺预处理的大鼠中则不然。米氮平(5 mg/kg/天,皮下注射,使用微型渗透泵)可增加雄性 Sprague-Dawley 大鼠蓝斑去甲肾上腺素 (NA) 神经元的自发放电活动。米氮平可拮抗低剂量(10 mg/kg,静脉注射)α2-肾上腺素受体激动剂可乐定对电刺激有效性的增强作用和高剂量(100 mg/kg,静脉注射)的降低作用。上行 5-HT 通路抑制背侧海马 CA3 锥体神经元的放电活动。 Mirtazapine (5 mg/kg sc) 仅轻微影响纹状体中的 DOPAC 和高香草酸水平,几乎不影响自由活动大鼠的 5-HT 释放,但明显增加 5-羟基吲哚乙酸。
|
| 酶活实验 |
比较了1,2,3,4,10,14b-六氢-2-甲基吡嗪并[2,1-a]吡啶并[2,3-c][2]苯并氮杂卓[+/-)Org 3770和相关抗抑郁药物mianserin的神经化学和自主药理学特征。与mianserin(pKi=7.4)相比,Org 3777(+/-)对[3H]去甲肾上腺素([3H]NA)的体外摄取影响较弱(pKi=5.6)。(+/-)Org 3770和米安色林都促进了皮质切片中[3H]NA的释放。α2肾上腺素受体介导的NA对[3H]NA或[3H]血清素([3H]5-HT)释放的影响被(+)Org 3770拮抗,pKi值分别为8.4和8.1。然而,(-)Org 3770仅拮抗NA对[3H]5-HT释放的影响(pA2=7.7)。(+/-)Org 3770和米安色林以相同的亲和力(pKi=7.0)抑制了[3H]劳沃尔辛与α2-肾上腺素受体的结合,而(+/-”Org 3777”(pKi=6.4)对[3H]哌唑嗪与α1-肾上腺素受体结合的影响小于米安色琳”(pKi=7.1)。在大鼠输精管中的α1-和α2-肾上腺素受体也发现了类似的差异。(+/-)Org 3770(pKi=8.1)对[3H]米安色林与5-HT2受体的结合的阻断作用不如米安色林强(pKi=4.4),而(+/-”Org 3777(pKi=9.3)对[3K]美吡拉敏与组胺-1受体的结合作用比米安色兰强(pKi=8.75)。[3H]奎核环烷基苯甲酸酯与毒蕈碱胆碱能受体的结合被(+/-)Org 3770(pKi=6.1)和米安色林(pKi=6.3)同等阻断。在分离器官中获得了类胰蛋白酶-D、组胺-1和毒蕈碱胆碱能受体的类似数据。阻断α2肾上腺素受体在米安色林和(+/-)Org 3770治疗抑郁症的疗效中起着重要作用,可能排除了抑制NA摄取的作用[2]。
|
| 细胞实验 |
米氮平对人单核细胞和CD4 T细胞在体外产生细胞因子/趋化因子的影响[3]
使用autoMACS分离机和autoMACS CD14+阳性选择试剂盒从健康供体外周血中分离CD14+单核细胞。将CD14+细胞接种到添加了10%FBS、1 mM丙酮酸钠、2 mM l-谷氨酰胺和100单位/ml青霉素和链霉素以及非必需氨基酸(NEAA)的500μl RPMI 1640培养基中的24孔组织培养板(密度为1×106个细胞/孔)中。孵育4小时(5%CO2,37°C)后,通过洗涤去除非贴壁细胞,并将500μl预热的完全新鲜培养基加入孔中。指定的孔用米氮平(10μM)或赋形剂(0.2μl/ml DMSO)处理。一小时后,将Con A(5μg/ml)或载体加入指定的孔中,细胞再培养24小时。收集上清液并储存在-80°C下,直至检测细胞因子/趋化因子水平(以pg/ml表示) 使用EasySep™人CD4+T细胞分离试剂盒从健康供体外周血中分离CD4+T细胞。流式细胞术检测的分离细胞纯度>97%。细胞在24孔板(密度106个细胞/孔)中在500μl RPMI 1640培养基中培养,该培养基补充了10%FBS、1 mM丙酮酸钠、2 mM l-谷氨酰胺和100单位/ml青霉素和链霉素以及非必需氨基酸(NEAA)。指定的孔用米氮平(10μM)或赋形剂(0.2μl/ml DMSO)处理。一小时后,将Con A(5μg/ml)或载体加入指定的孔中,细胞再培养24小时。收集上清液并储存在-80°C下,直至检测细胞因子水平。根据制造商的方案,使用人MILLIPEX试剂盒在培养上清液中测量人IL-10、IL-4和IFNγ。使用Luminex 100系统进行多路复用分析 。 |
| 动物实验 |
Male C57BL/6 mice (8-10 week old) treated with concanavalin A (Con A)
1 mg/kg, 10 mg/kg, and 20 mg/kg Intraperitoneal injection; once Mirtazapine Treatment and Con A Hepatitis Severity[3] To delineate the impact of mirtazapine treatment in Con A hepatitis, mice were treated 1 h prior to Con A treatment with mirtazapine 1–20 mg/kg intraperitoneally (ip). Blood and liver samples were collected under isoflurane anesthesia 16 h post-Con A treatment (unless otherwise noted) to assess liver injury biochemically (plasma alanine aminotransferase [ALT] activity; measured using Roche-Hitachi Modular-P800 apparatus) and histologically using formalin-fixed liver tissue slices stained with Hematoxylin and Eosin (H&E). Extent of liver parenchymal necrosis was quantitated as previously described using Image J software and an Olympus XC10 camera (acquired using the Olympus VS-ASW software package; original magnification x400). In additional experiments, mirtazapine (20 mg/kg ip) was administered 2 h after Con A treatment (i.e., therapeutically) and mice sacrificed 16 h later and severity of liver injury determined by ALT measurement. In further experiments, the impact of specifically blocking individual receptors known to be impacted by mirtazapine treatment (i.e., 5HT2a, 5HT2c, 5HT3, and H1; also 5HT1a receptor) on the severity of Con A hepatitis was determined by ALT measurement. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The absorption of this drug is rapid and complete. Due to first pass metabolism in the liver and metabolism in the gut wall, absolute bioavailability is about 50%. Peak blood concentrations are attained within about 2 hours after an oral dose. Food has little effect on the absorption of mirtazapine, and no dose adjustment is required if it is taken with food. Steady-state levels are achieved by about 5 days after the initial dose. Mirtazapine pharmacokinetics vary across gender and age range. Females and the elderly population have been shown to have higher blood concentrations in comparison to males and younger adults. This drug is mainly excreted by the kidney. It is 75% eliminated in the urine and 15% eliminated in the feces. The volume of distribution after an oral steady-state dose was measured to be 107 ± 42L in a pharmacokinetic study. Total body clearance in males was found to be 31 L/h in a clinical pharmacokinetics study after intravenous administration. **Clearance in elderly patients* Mirtazapine clearance is slower in the elderly than in younger subjects. Exercise caution when this drug is given to elderly patients. In a clinical trial, elderly males showed a marked decrease in mirtazapine clearance when compared to young males taking the same dose. This difference was less significant when clearance was compared between elderly females and younger females taking mirtazapine. **Clearance in hepatic and renal impairment** Patients with hepatic and renal impairment have decreased rates of clearance and dosage adjustments may be necessary for these patients. Moderate renal impairment and hepatic impairment cause about a 30% decrease in mirtazapine clearance. Severe renal impairment leads to a 50% decrease in mirtazapine clearance. Metabolism / Metabolites Mirtazapine is heavily metabolized in humans. Demethylation and hydroxylation and subsequent glucuronide conjugation are the major pathways by which mirtazapine is metabolized. Data from in vitro studies on human liver microsomes show that cytochrome 2D6 and 1A2 lead to the formation of the _8-hydroxy metabolite_ of mirtazapine. The CYP3A enzyme metabolizes this drug into its _N-desmethyl and N-oxide_ metabolites. There are various other unconjugated metabolites of this drug that are pharmacologically active, but are measured in the blood at limited concentrations. Mirtazapine has known human metabolites that include Mirtazapine N-oxide, N-Desmethylmirtazapine, and 8-hydroxy-mirtazapine. Mirtazapine is extensively metabolized by demethylation and hydroxylation followed by glucuronide conjugation. Cytochrome P450 2D6 and cytochrome P450 1A2 are involved in formation of the 8-hydroxy metabolite of mirtazapine, and cytochrome P450 3A4 is responsible for the formation of the N-desmethyl and N-oxide metabolites. Several metabolites possess pharmacological activity, but plasma levels are very low. Route of Elimination: This drug is known to be substantially excreted by the kidney (75%). Half Life: 20-40 hours Biological Half-Life 20-40 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Mirtazapine acts as an antagonist at central pre-synaptic alpha(2)-receptors, inhibiting negative feedback to the presynaptic nerve and causing an increase in NE release. Blockade of heteroreceptors, alpha(2)-receptors contained in serotenergic neurons, enhances the release of 5-HT, increasing the interactions between 5-HT and 5-HT1 receptors and contributing to the anxiolytic effects of mirtazapine. Mirtazapine also acts as a weak antagonist at 5-HT1 receptors and as a potent antagonist at 5-HT2 (particularly subtypes 2A and 2C) and 5-HT3 receptors. Blockade of these receptors may explain the lower incidence of adverse effects such as anxiety, insomnia, and nausea. Mirtazapine also exhibits significant antagonism at H1-receptors, resulting in sedation. Mirtazapine has no effects on the reuptake of either NE or 5-HT and has only minimal activity at dopaminergic and muscarinic receptors. Toxicity Data LD50: 600-720mg/kg (oral, mice) (L1855) LD50: 320-490mg/kg (oral, rat) (L1855) |
| 参考文献 |
|
| 其他信息 |
Pharmacodynamics
**General effects and a note on suicidality** Mirtazapine is effective in treating moderate to severe depression and treats many symptoms normally associated with this condition. These symptoms may include disturbed sleep, lack of appetite, and anhedonia, in addition to anxiety.. It is important to note that suicidal ideation and behavior may emerge or increase during treatment with mirtazapine, as with any other antidepressant. This risk is especially pronounced in younger individuals. Patients, medical professionals, and families should monitor for suicidal thoughts, worsening depression, anxiety, agitation, sleep changes, irritable behavior, aggression, impulsivity, restlessness, and other unusual behavior when this drug is taken or the dose is adjusted. Do not administer mirtazapine to children. When deciding to prescribe this drug, carefully consider the increased risk of suicidal thoughts and behavior, especially in young adults. **Effects on appetite and weight gain** In addition to the above effects, mirtazapine exerts stimulating effects on appetite, and has been used for increasing appetite and decreasing nausea in cancer patients. Some studies and case reports have shown that this drug improves eating habits and weight gain in patients suffering from anorexia nervosa when administered in conjunction with psychotherapy and/or other psychotropic drugs. In a clinical trial, women with depression experienced a clinically significant mean increase in body weight, fat mass, and concentrations of leptin when treated with mirtazapine for a 6-week period, with a lack of effect on glucose homeostasis. **Effects on sleep** The use of mirtazapine to treat disordered sleep has been leveraged from its tendency to cause somnolence, which is a frequently experienced adverse effect by patients taking this drug. Mirtazapine has been shown to exert beneficial effects on sleep latency, duration, and quality due to its sedating properties. Insomnia is a common occurrence in patients with depression, and mirtazapine has been found to be efficacious in treating this condition. |
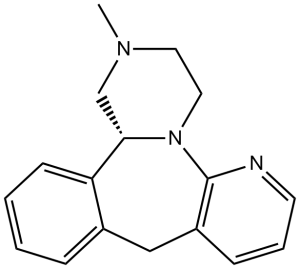
| 分子式 |
C17H19N3
|
|
|---|---|---|
| 分子量 |
265.35
|
|
| 精确质量 |
265.157
|
|
| 元素分析 |
C, 76.95; H, 7.22; N, 15.84
|
|
| CAS号 |
85650-52-8
|
|
| 相关CAS号 |
(S)-Mirtazapine; 61337-87-9; (S)-Mirtazapine-d3; (R)-Mirtazapine; 61364-37-2; Mirtazapine-d3; 1216678-68-0; Mirtazapine-d4; 1215898-55-7; (R)-Mirtazapine-d3;
85650-52-8; 61337-67-5 (deleted); 1448014-35-4 (HCl); 207516-99-2 (HCl); 207516-99-2 (2HCl); 868363-97-7 (HBr); 868528-74-9 (HBr); 341512-89-8 (hemihydrate)
|
|
| PubChem CID |
4205
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
432.4±45.0 °C at 760 mmHg
|
|
| 熔点 |
114-116ºC
|
|
| 闪点 |
215.3±28.7 °C
|
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
|
| 折射率 |
1.668
|
|
| LogP |
2.75
|
|
| tPSA |
19.37
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
0
|
|
| 重原子数目 |
20
|
|
| 分子复杂度/Complexity |
345
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
N1C2N3C(C4C(CC=2C=CC=1)=CC=CC=4)CN(C)CC3
|
|
| InChi Key |
RONZAEMNMFQXRA-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C17H19N3/c1-19-9-10-20-16(12-19)15-7-3-2-5-13(15)11-14-6-4-8-18-17(14)20/h2-8,16H,9-12H2,1H3
|
|
| 化学名 |
5-methyl-2,5,19-triazatetracyclo[13.4.0.02,7.08,13]nonadeca-1(15),8,10,12,16,18-hexaene
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.42 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.42 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (9.42 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7686 mL | 18.8430 mL | 37.6861 mL | |
| 5 mM | 0.7537 mL | 3.7686 mL | 7.5372 mL | |
| 10 mM | 0.3769 mL | 1.8843 mL | 3.7686 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Comparing Olanzapine and Mirtazapine in the Improvement of Unintentional Weight Loss for Patients with Advanced Stage Cancer
CTID: NCT05170919
Phase: Phase 2 Status: Enrolling by invitation
Date: 2024-09-19
 |
|---|