| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g | |||
| Other Sizes |

| 靶点 |
α1-adrenergic receptor; α2-adrenergic receptor; Beta-1 adrenergic receptor; Microbial Metabolite; Human Endogenous Metabolite
|
|---|---|
| 体外研究 (In Vitro) |
去甲肾上腺素 (NE) 通常被认为是 β1-亚型 β1- 开始素能激动剂,β2- 开始素能接收。去甲肾上腺素 (NE) 在上一个下浓度也对 β2- 开始素能接收具有直接活性[1]。来自腹股沟脂肪垫(iWA)或肩肩间脂肪垫(BA)来自新生野生型C57BL/6J 小鼠中分离并培养。为了检查激活AT2对β-首先素能通报信号的影响,首先评估 cAMP 产生对去甲肾上腺素 (NE,10 μM) 有或没有 CGP (10 nM) 联合处理的反应。 去甲肾上腺素 (NE) 增加 cAMP 正如在 iWA 中预期的那样,CGP 不会改变这种效果 去甲肾上腺素 (NE) ) 也已知会诱导脂肪减少,并且需要释放的培养基来功能性激活 UCP1 蛋白质并刺激热量产生。在小鼠 iWA 中,去甲肾上腺素 (NE) 处理后 Ser133 处的 CREB 磷酸化增加,并且与 CGP 联合处理明显分开[3]。
|
| 体内研究 (In Vivo) |
去甲肾上腺素可用于动物建模,构建动物模型。
|
| 细胞实验 |
皮下前脂肪细胞通过 TERT 和 HPV E6/E7 永生化,该细胞来自一名非糖尿病的 38 岁女性捐赠者。为了促进当前的研究,使用环克隆来分离具有恒定分化能力的稳定二倍体克隆(称为克隆 B)。前脂肪细胞PGM2培养基用于培养细胞。在含有地塞米松、IBMX、吲哚美辛和额外胰岛素的分化培养基中孵育,一旦细胞汇合,就会诱导细胞分化。十天内,细胞分化。用于治疗的培养基在更换为PGM2培养基一天后更换为无血清培养基过夜。 NE (10 μM)、CGP (10 nM)、媒介物或 NE 和 CGP 是对脂肪细胞进行六小时的治疗[2]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Norepinephrine localizes mainly in sympathetic nervous tissue. The drug crosses the placenta but not the blood-brain barrier. Orally ingested norepinephrine is destroyed in the GI tract, and the drug is poorly absorbed after subcutaneous injection. After IV administration, a pressor response occurs rapidly. The drug has a short duration of action, and the pressor action stops within 1-2 minutes after the infusion is discontinued. Norepinephrine, like epinephrine, is ineffective when given orally and is absorbed poorly from sites of subcutaneous injection. It is rapidly inactivated in the body by the same enzymes that methylate and oxidatively deaminate epinephrine. Small amounts normally are found in the urine. The excretion rate may be greatly increased in patients with pheochromocytoma. Metabolism / Metabolites The pharmacologic actions of norepinephrine are terminated primarily by uptake and metabolism in sympathetic nerve endings. The drug is metabolized in the liver and other tissues by a combination of reactions involving the enzymes catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO). The major metabolites are normetanephrine and 3-methoxy-4-hydroxy mandelic acid (vanillylmandelic acid, VMA), both of which are inactive. Other inactive metabolites include 3-methoxy-4-hydroxyphenylglycol, 3,4-dihydroxymandelic acid, and 3,4-dihydroxyphenylglycol. Norepinephrine metabolites are excreted in urine primarily as the sulfate conjugates and, to a lesser extent, as the glucuronide conjugates. Only small quantities of norepinephrine are excreted unchanged. Uremic toxins tend to accumulate in the blood either through dietary excess or through poor filtration by the kidneys. Most uremic toxins are metabolic waste products and are normally excreted in the urine or feces. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Uremic toxins such as noradrenalin are actively transported into the kidneys via organic ion transporters (especially OAT3). Increased levels of uremic toxins can stimulate the production of reactive oxygen species. This seems to be mediated by the direct binding or inhibition by uremic toxins of the enzyme NADPH oxidase (especially NOX4 which is abundant in the kidneys and heart) (A7868). Reactive oxygen species can induce several different DNA methyltransferases (DNMTs) which are involved in the silencing of a protein known as KLOTHO. KLOTHO has been identified as having important roles in anti-aging, mineral metabolism, and vitamin D metabolism. A number of studies have indicated that KLOTHO mRNA and protein levels are reduced during acute or chronic kidney diseases in response to high local levels of reactive oxygen species (A7869). Norepinephrine functions as a peripheral vasoconstrictor by acting on alpha-adrenergic receptors. It is also an inotropic stimulator of the heart and dilator of coronary arteries as a result of it's activity at the beta-adrenergic receptors. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of norepinephrine during breastfeeding. Because of its poor oral bioavailability and short half-life, any norepinephrine in milk is unlikely to affect the infant. High intravenous doses of norepinephrine might reduce milk production or milk letdown as well as decrease the concentration of beta-casein in milk. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Norepinephrine inhibits the synthesis of beta-casein via stimulation of adrenergic beta-2 receptors. Animal data indicate that norepinephrine can decrease serum prolactin and reduce milk production, as well as inhibit the release of oxytocin, which inhibits milk ejection. Interactions Cyclopropane and halothane anesthetics increase cardiac autonomic irritability and therefore seem to sensitize the myocardium to the action of intravenously administered epinephrine or norepinephrine bitartrate injection. Hence, the use of norepinephrine bitartrate injection during cyclopropane and halothane anesthesia is generally considered contraindicated because of the risk of producing ventricular tachycardia or fibrillation. Enhanced pressor response may occur in patients taking monoamine oxidase (MAO) inhibitors owing to inhibition of neuronal metabolic degradation. Administration of furosemide or other diuretics may decrease arterial responsiveness to pressor drugs such as norepinephrine. Tricyclic antidepressants (e.g., imipramine), some antihistamines (especially diphenhydramine, tripelennamine, and dexchlorpheniramine), parenteral ergot alkaloids, guanethidine, or methyldopa may potentiate the pressor effects of norepinephrine, resulting in severe, prolonged hypertension. Norepinephrine should be given cautiously and in small doses to patients receiving these drugs. Potentiation may result from inhibition of tissue uptake of norepinephrine or by increased adrenoreceptor sensitivity to the drug. Monoamine oxidase (MAO) is one of the enzymes responsible for norepinephrine metabolism. Although some clinicians have reported that MAO inhibitors do not appear to potentiate the effects of norepinephrine to a clinically important extent, the manufacturer states that norepinephrine should be administered with extreme caution to patients receiving an MAO inhibitor because severe, prolonged hypertension may result. For more Interactions (Complete) data for Norepinephrine (8 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat iv 100 ug/kg LD50 Mouse oral 20 mg/kg LD50 Mouse ip 6 mg/kg LD50 Mouse sc 5 mg/kg LD50 Mouse iv 550 ug/kg |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Norepinephrine is used to produce vasoconstriction and cardiac stimulation as an adjunct to correct hemodynamic imbalances in the treatment of shock that persists after adequate fluid volume replacement. /Included in US product label/ Epinephrine is the drug of choice in the emergency treatment of severe acute anaphylactic reactions, including anaphylactic shock. Once adequate ventilation is assured, maintenance of blood pressure in patients with anaphylactic shock may be achieved with other pressor agents, such as norepinephrine. /Included in US product label/ In hypotension associated with myocardial infarction, cautious administration of norepinephrine may be of value and some clinicians consider it to be the pressor drug of choice. However, this type of shock generally has a poor prognosis even when pressor agents are used, and norepinephrine-induced increases in myocardial oxygen demand and the work of the heart may outweigh the beneficial effects of the drug. In addition, cardiac arrhythmias due to the drug are more likely to occur in patients with myocardial infarction. If severe congestive heart failure is also present, dopamine may be preferable because it increases renal blood flow as well as stroke volume. If peripheral vascular resistance is elevated, isoproterenol may be used in conjunction with norepinephrine, but dosage of both drugs must be carefully adjusted according to the specific hemodynamic imbalances present. /Included in US product label/ Norepinephrine may be used to treat hypotension occurring during spinal anesthesia, but other vasopressors having a longer duration of action and which can be administered IM such as metaraminol, methoxamine, or phenylephrine are more commonly used. Norepinephrine may be used to treat hypotension occurring during general anesthesia; however, the possibility of cardiac arrhythmias should be considered. /Included in US product label/ For more Therapeutic Uses (Complete) data for Norepinephrine (7 total), please visit the HSDB record page. Drug Warnings Norepinephrine can cause severe peripheral and visceral vasoconstriction, reduced blood flow to vital organs, decreased renal perfusion and therefore decreased urine output, tissue hypoxia, and metabolic acidosis. These effects are most likely to occur in hypovolemic patients. In addition, prolonged use of norepinephrine may cause plasma volume depletion which may result in perpetuation of the shock state or recurrence of hypotension when the drug is discontinued. Prolonged administration of norepinephrine has caused edema, hemorrhage, focal myocarditis, subpericardial hemorrhage, necrosis of the intestine, or hepatic and renal necrosis. These effects have generally occurred in patients with severe shock and it is not clear if the drug or the shock state itself was the cause. Norepinephrine can cause tissue necrosis and sloughing at the site of injection as a result of local vasoconstriction. Impairment of circulation and sloughing of tissue may also occur without obvious extravasation. Gangrene of the extremities has been reported rarely and has occurred in a lower extremity when norepinephrine was injected into an ankle vein. Norepinephrine increases myocardial oxygen consumption and the work of the heart. Cardiac output may be decreased following prolonged use of the drug or administration of large doses because venous return to the heart may be diminished because of increased peripheral vascular resistance. Decreased cardiac output may be especially harmful to elderly patients or those with initially poor cerebral or coronary circulation. Norepinephrine may cause palpitation and bradycardia as well as potentially fatal cardiac arrhythmias, including ventricular tachycardia, bigeminal rhythm, nodal rhythm, atrioventricular dissociation, and fibrillation. Bradycardia may be treated by administration of atropine. Arrhythmias are especially likely to occur in patients with acute myocardial infarction, hypoxia, or hypercapnia, or those receiving other drugs which may increase cardiac irritability such as cyclopropane or halogenated hydrocarbon general anesthetics. For more Drug Warnings (Complete) data for Norepinephrine (19 total), please visit the HSDB record page. Pharmacodynamics Noradrenaline acts on both alpha-1 and alpha-2 adrenergic receptors to cause vasoconstriction. Its effect in-vitro is often limited to the increasing of blood pressure through antagonising alpha-1 and alpha-2 receptors and causing a resultant increase in systemic vascular resistance. |
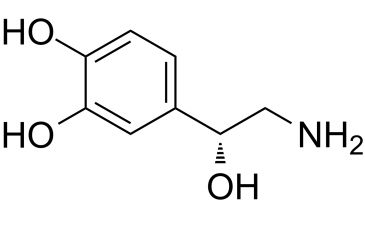
| 分子式 |
C8H11NO3
|
|---|---|
| 分子量 |
169.1778
|
| 精确质量 |
169.073
|
| 元素分析 |
C, 56.80; H, 6.55; N, 8.28; O, 28.37
|
| CAS号 |
51-41-2
|
| 相关CAS号 |
Norepinephrine hydrochloride; 329-56-6; Norepinephrine bitartrate monohydrate; 108341-18-0; Norepinephrine tartrate; 51-40-1; (Rac)-Norepinephrine-d3 (formate)
|
| PubChem CID |
439260
|
| 外观&性状 |
White to yellow solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
442.6±40.0 °C at 760 mmHg
|
| 熔点 |
220-230°C
|
| 闪点 |
221.5±27.3 °C
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
| 折射率 |
1.659
|
| LogP |
-0.88
|
| tPSA |
86.71
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
12
|
| 分子复杂度/Complexity |
142
|
| 定义原子立体中心数目 |
1
|
| SMILES |
OC1=CC=C([C@@H](O)CN)C=C1O
|
| InChi Key |
SFLSHLFXELFNJZ-QMMMGPOBSA-N
|
| InChi Code |
InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2/t8-/m0/s1
|
| 化学名 |
4-[(1R)-2-amino-1-hydroxyethyl]benzene-1,2-diol
|
| 别名 |
Norepinephrine; Noradrenaline; Noradrenalin; Levarterenol; Levophed Arterenol
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: (1). 本产品在运输和储存过程中需避光。 (2). 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~25 mg/mL (~147.8 mM)
H2O: < 0.1 mg/mL
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (12.29 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (12.29 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (12.29 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.9109 mL | 29.5543 mL | 59.1086 mL | |
| 5 mM | 1.1822 mL | 5.9109 mL | 11.8217 mL | |
| 10 mM | 0.5911 mL | 2.9554 mL | 5.9109 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Blood PREssure Augmentation in Large-vessel Occlusion Stroke Study
CTID: NCT04218773
PhaseEarly Phase 1 Status: Enrolling by invitation
Date: 2024-10-03
|
|
|
|