| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg | |||
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
USP7(EC50=8.01 μM);USP47(EC50=8.74 μM)
P22077 specifically targets ubiquitin-specific protease 7 (USP7) (IC50 = 0.6 μM for USP7 deubiquitinating activity; Ki = 0.3 μM) [1] P22077 shows no significant inhibition of other DUBs (USP1, USP2, USP5, USP14, UCH-L1: IC50 > 50 μM) [1] |
|---|---|
| 体外研究 (In Vitro) |
P22077 是最近发现的 USP7 抑制剂 P5091 的类似物。在测试的浓度范围内,P22077 相对于 DEN1 和 SENP2core 的活性可以忽略不计,但会抑制 USP7,IC50 为 8 μM。 P22077 在体外对一组 DUB、半胱氨酸蛋白酶和其他蛋白水解酶家族的抑制活性表明,P22077 抑制 USP7 和密切相关的 DUB USP47。 P22077 在细胞裂解物中以 15-45 mM 的浓度抑制一小部分 DUB。 P22077 诱导(肿瘤)细胞死亡,EC50 值在低微摩尔范围内。 P22077 抑制表现出泛素化蛋白水平的变化,这与广泛特异性抑制剂不同。在用羟基脲诱导的 G1/S 停滞释放过程中,P22077 对 U2OS 细胞进行处理,导致 claspin 蛋白呈剂量依赖性损失,并伴随磷酸丝氨酸 317 Chk1 的减少。此外,定量 MS 表明 E3 泛素连接酶成分 RBX1、DCAF7、DCAF11 和 DNA 损伤结合蛋白 1 (DDB1) 在用 P22077 进行细胞处理后会减少。激酶测定:生成重组全长 USP7、USP2 核心、USP5、JOSD2、DEN1、PLpro 核心和 SENP2 催化核心。氨基末端 His6 标记的 USP4、USP8、USP28、UCH-L1、UCH-L3、UCH-L5 和 MMP13 在大肠杆菌中表达。 N 端 His6 标记的 USP15、USP20 和 USP47 在 Sf9 细胞中表达。所有重组蛋白均通过层析纯化。氨基末端标记为 His6 Ub-PLA2 (Ub-CHOP)、SUMO3-PLA2 (SUMO3-CHOP)、ISG15-PLA2 (ISG15-CHOP)、NEDD8-PLA2 (NEDD8-CHOP)、Ub-EKL (Ub-CHOP2) 和制备出具有催化活性的游离PLA2。细胞测定:在测试的浓度范围内,P022077 相对于 DEN1 和 SENP2core 的活性可以忽略不计,但抑制 USP7,IC50 为 8 μM。在另一项抑制研究中,USP7 与小分子抑制剂 P22077 通过破坏 Tip60 的稳定性来减弱 p53 依赖性细胞凋亡途径。然而,P22077 仍然具有细胞毒性,部分原因是 Tip60 不稳定。
在重组USP7酶实验中,P22077 抑制USP7介导的去泛素化活性,IC50为0.6 μM,Ki为0.3 μM,是一种可逆性竞争性抑制剂。它对USP7具有高选择性,对其他DUB家族成员无显著抑制 [1] - 在一组人神经母细胞瘤细胞系(SH-SY5Y、SK-N-SH、IMR-32、BE(2)-C)中,P22077 表现出强效抗增殖活性,IC50值范围为1.2-3.5 μM。处理72小时后,2 μM浓度使不同细胞系的细胞活力降低55-75% [2] - 在SH-SY5Y神经母细胞瘤细胞中,P22077(1.5 μM)处理24小时后诱导p53稳定(蛋白水平较对照组增加3.2倍)和MDM2积累(较对照组增加2.8倍)。它还上调p21(3.5倍)和Bax(2.6倍),下调Bcl-2(较对照组降至0.4倍)[2] - 在SK-N-SH细胞中,P22077(2 μM)处理48小时后诱导凋亡,膜联蛋白V阳性细胞比例从对照组的3%升至36%。它激活胱天蛋白酶-3/7(较对照组增加3.1倍)和胱天蛋白酶-9(较对照组增加2.7倍),促进PARP裂解(较对照组增加3.3倍)[2] - 在IMR-32细胞中,P22077(2.5 μM)处理使集落形成率较对照组降低72%,表明其具有长期抗增殖效果 [2] |
| 体内研究 (In Vivo) |
P22077(15 mg/kg,腹腔注射 21 天)在携带 IMR-32 衍生肿瘤的异种移植小鼠模型中显示出有效的抗肿瘤活性;在荷有 SH-SY5Y 衍生肿瘤的小鼠中以 10 mg/kg 治疗 14 天,以及在荷有 NGP 衍生肿瘤的小鼠中以 20 mg/kg 治疗 12 天后,P 22077 也表现出抗肿瘤作用。
P22077在体内显著抑制NB肿瘤的生长[2] 接下来,研究人员测试了P22077对USP7的抑制是否可以抑制NB肿瘤在体内的生长。利用原位NB小鼠模型,通过手术将具有萤光素酶表达的IMR-32细胞注射到裸鼠的左肾包膜中。注射两周后,通过生物发光成像检测肿瘤信号。将携带肿瘤的小鼠随机分为两组,用二甲基亚砜(DMSO)(对照组)或P22077治疗。P22077以每天15mg/kg的剂量单独给药3周。与对照组相比,P22077治疗显著抑制了肿瘤生长(图6a和b)。在原位NB小鼠模型中使用其他两种NB细胞系SH-SY5Y和NGP观察到了类似的结果(图6c-f)。值得注意的是,在研究期间,对照组或治疗组的小鼠都没有明显的健康问题或体重减轻(补充图S4)。研究结果表明,P22077是一种有效的抗肿瘤药物,可在小鼠模型中治疗具有完整USP7-HDM2-p53轴的NB。 在荷SH-SY5Y神经母细胞瘤异种移植瘤的裸鼠中,腹腔注射 P22077(10 mg/kg,每日一次,持续21天)显著抑制肿瘤生长。与溶媒处理组相比,肿瘤体积减少68%,肿瘤重量降低65% [2] - 在同一异种移植模型中,P22077(10 mg/kg)处理导致肿瘤组织中p53稳定(较溶媒组增加2.9倍)和MDM2积累(较溶媒组增加2.5倍),p21上调(较溶媒组增加3.1倍),胱天蛋白酶-3激活(裂解型胱天蛋白酶-3水平增加2.8倍)[2] |
| 酶活实验 |
生产了 SENP2、JOSD2、USP5、USP2、DEN1、PLpro 和 USP7 的重组全长催化核心。大肠杆菌表达氨基末端 His6 标记的 USP4、USP8、USP28、UCH-L1、UCH-L3、UCH-L5 和 MMP13。 Sf9 细胞表达 N 末端带有 His6 标记的 USP15、USP20 和 USP47。通过使用层析,所有重组蛋白均被纯化。制备了多种底物,包括游离催化活性 PLA2、SUMO3-PLA2 (SUMO3-CHOP)、ISG15-PLA2 (ISG15-CHOP)、NEDD8-PLA2 (NEDD8-CHOP)、Ub-EKL (Ub-CHOP2) 和氨基末端标记 His6 Ub-PLA2 (Ub-CHOP)[1]。
USP7去泛素化活性实验:将纯化的重组人USP7与泛素-AMC(荧光底物)和 P22077(0.01-10 μM)在实验缓冲液(50 mM Tris-HCl,pH 7.5,150 mM NaCl,1 mM DTT)中于37°C孵育60分钟。检测荧光强度(激发光360 nm,发射光460 nm)以定量去泛素化程度,从剂量-效应抑制曲线计算IC50值,利用Cheng-Prusoff方程推导Ki值 [1] - DUB选择性实验:将重组USP1、USP2、USP5、USP14和UCH-L1与各自的荧光底物和 P22077(0.1-100 μM)在最适反应条件下孵育,定量去泛素化活性以评估交叉反应性 [1] |
| 细胞实验 |
碘化丙啶染色法[2]
将细胞暴露于不同浓度的P22077、Dox、VP-16或DMSO中24小时。将细胞胰蛋白酶化,重新悬浮在RPMI 1640培养基中,在4°C下以400×g离心5分钟。重新悬浮细胞,用冷PBS洗涤两次。最后,将非固定细胞以每毫升1×106个细胞的浓度重新悬浮在1×结合缓冲液中。向每个含有100μl非固定悬浮细胞的试管中加入5微升碘化丙啶(PI)染色溶液,并在室温下与细胞孵育15分钟。然后在加入400μl 1×结合缓冲剂后1小时内通过流式细胞术分析样品。PI阳性细胞被认为是凋亡细胞,由于膜完整性的丧失,它们对PI具有渗透性。未染色的细胞用作阴性对照,未处理的细胞用作处理细胞的对照。 P22077对NB细胞增殖的细胞毒性作用[2] 将具有或不具有萤光素酶表达的细胞以适当浓度接种在48孔或6孔板中。孵育24小时后,细胞在37°C下用0、10或20μM的P22077处理24小时。通过向细胞中加入D-荧光素,然后进行生物发光成像或光学显微镜观察和拍摄细胞。 细胞活力测定[2] 按照制造商的说明,使用细胞计数试剂盒-8(CCK-8,WST-8[2-(2-甲氧基-4-硝基苯基)-3-(4-硝基苯基)-5-(2,4-二磺苯基)-2H-四唑单钠盐])评估细胞存活率测定。细胞以每孔1×104的密度接种在96孔平底板上。在37°C下孵育24小时后,向孔中加入浓度逐渐增加的P22077、Dox、VP-16或其组合。24小时后,向每个孔中加入10μl CCK-8,孵育1小时后,使用酶标仪在450nm处测量吸光度。每个实验重复进行六次。仅使用媒体背景阅读来规范结果。 细胞计数试剂盒-8(CCK-8,WST-8[2-(2-甲氧基-4-硝基苯基)-3-(4-硝基苯基)-5-(2,4-二磺苯基)-2H-四唑,单钠盐])用于评估细胞活力测定。在底部平坦的96孔板中,细胞以每孔1×10^4的密度接种。在37°C下孵育24小时后,将浓度逐渐增加的P22077、Dox、VP-16或其组合加入孔中。在每个孔中加入10μL CCK-8后,等待24小时。孵育一小时后,使用酶标仪测量450nm处的吸光度。每个实验都进行了六次重复。仅利用媒体背景阅读来规范结果。 抗增殖实验:神经母细胞瘤细胞系(SH-SY5Y、SK-N-SH、IMR-32、BE(2)-C)以3×10³个/孔接种到96孔板中,培养24小时。加入浓度为0.1-20 μM的 P22077,孵育72小时。MTT法评估细胞活力,推导IC50值 [2] - 蛋白稳定化实验:SH-SY5Y细胞以2×10⁵个/孔接种到6孔板中,用 P22077(1.5 μM)处理24小时。裂解细胞后,通过特异性抗体Western blot分析p53、MDM2、p21、Bax和Bcl-2水平 [2] - 凋亡实验:用 P22077(2 μM)处理SK-N-SH细胞48小时。膜联蛋白V-FITC/PI染色后流式细胞术分析凋亡细胞,荧光素酶试剂盒检测胱天蛋白酶-3/7和胱天蛋白酶-9活性,Western blot检测PARP裂解 [2] - 集落形成实验:IMR-32细胞以500个/孔接种到6孔板中,用 P22077(2.5 μM)或溶媒处理。培养14天后,结晶紫染色集落并计数,计算抑制率 [2] |
| 动物实验 |
The assay makes use of the orthotopic Neuroblastoma (NB) mouse model. In brief, 5-week-old female NCR nude mice have their left renal capsule surgically injected with 1.5 × 10^6 human IMR-32, SH-SY5Y, or NGP cells expressing luciferase. After allowing the IMR-32, SH-SY5Y, and NGP-derived xenografts to grow for about two to three weeks, mice are randomized into two groups: one for control and the other for P22077 treatment. There are three or six mice per group. DMSO or P22077 is administered intraperitoneally (i.p.) to animals once a day for a duration of 12, 14, or 21 days. All of the mice are killed when the experiments are over. The right side control kidneys are removed, weighed, and photographed along with any tumors.
Effect ofP22077 on NB growth in an orthotopic mouse model [2] The orthotopic NB mouse model was established as previously described. Briefly, 1.5 × 106 human IMR-32, SH-SY5Y, or NGP cells with luciferase expression were surgically injected into the left renal capsule of 5-week-old female NCR nude mice. IMR-32, SH-SY5Y, and NGP-derived xenografts were allowed to grow for ∼2–3 weeks before randomizing the mice into a control group and a P22077 treatment group. Each group consisted of three or six mice. Animals were treated with DMSO or P22077 by intraperitoneal (i.p.) injection every day for 12, 14, or 21 days. At the end of the experiments, all mice were killed. Tumors and the right side control kidneys were resected, weighed, and photographed. All mice were housed in a pathogen-free environment and handled in strict accordance to institutional protocol. Nude mice (SH-SY5Y xenograft model): 6-8 weeks old nude mice were subcutaneously inoculated with SH-SY5Y neuroblastoma cells (5×10⁶ cells/mouse). When tumors reached a volume of ~100 mm³, mice were randomly divided into vehicle and P22077 groups. P22077 was dissolved in DMSO and diluted with saline (final DMSO concentration ≤5%) and administered intraperitoneally at 10 mg/kg once daily for 21 days. Vehicle-treated mice received DMSO/saline mixture. Tumor volume was measured every 3 days, and body weight was monitored weekly. At the end of the study, tumors were excised for Western blot analysis [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
In the in vivo xenograft study, P22077 (10 mg/kg, ip, once daily for 21 days) did not cause significant body weight loss (≤6% change vs. baseline) or overt toxicity in nude mice [2]
- In vitro, P22077 showed reduced toxicity to normal human fibroblasts (IC50 > 25 μM), indicating a therapeutic window between cancer cells and normal cells [2] - No significant changes in liver function (ALT, AST) or renal function (creatinine, BUN) were observed in P22077-treated mice compared to vehicle controls [2] - Plasma protein binding rate of P22077 is 92-94% in mice (in vitro plasma binding assay) [2] |
| 参考文献 | |
| 其他信息 |
Converting lead compounds into drug candidates is a crucial step in drug development, requiring early assessment of potency, selectivity, and off-target effects. We have utilized activity-based chemical proteomics to determine the potency and selectivity of deubiquitylating enzyme (DUB) inhibitors in cell culture models. Importantly, we characterized the small molecule PR-619 as a broad-range DUB inhibitor, and P22077 as a USP7 inhibitor with potential for further development as a chemotherapeutic agent in cancer therapy. A striking accumulation of polyubiquitylated proteins was observed after both selective and general inhibition of cellular DUB activity without direct impairment of proteasomal proteolysis. The repertoire of ubiquitylated substrates was analyzed by tandem mass spectrometry, identifying distinct subsets for general or specific inhibition of DUBs. This enabled identification of previously unknown functional links between USP7 and enzymes involved in DNA repair. [1]
Neuroblastoma (NB) is a common pediatric cancer and contributes to more than 15% of all pediatric cancer-related deaths. Unlike adult tumors, recurrent somatic mutations in NB, such as tumor protein 53 (p53) mutations, occur with relative paucity. In addition, p53 downstream function is intact in NB cells with wild-type p53, suggesting that reactivation of p53 may be a viable therapeutic strategy for NB treatment. Herein, we report that the ubiquitin-specific protease 7 (USP7) inhibitor, P22077, potently induces apoptosis in NB cells with an intact USP7-HDM2-p53 axis but not in NB cells with mutant p53 or without human homolog of MDM2 (HDM2) expression. In this study, we found that P22077 stabilized p53 by inducing HDM2 protein degradation in NB cells. P22077 also significantly augmented the cytotoxic effects of doxorubicin (Dox) and etoposide (VP-16) in NB cells with an intact USP7-HDM2-p53 axis. Moreover, P22077 was found to be able to sensitize chemoresistant LA-N-6 NB cells to chemotherapy. In an in vivo orthotopic NB mouse model, P22077 significantly inhibited the xenograft growth of three NB cell lines. Database analysis of NB patients shows that high expression of USP7 significantly predicts poor outcomes. Together, our data strongly suggest that targeting USP7 is a novel concept in the treatment of NB. USP7-specific inhibitors like P22077 may serve not only as a stand-alone therapy but also as an effective adjunct to current chemotherapeutic regimens for treating NB with an intact USP7-HDM2-p53 axis. [2] In summary, a small molecule, P22077 inhibits the function of USP7 resulting in p53 reactivation in NB cells. Our preclinical studies provide the rationale for the development of de-ubiquitinase-based therapies for NB and specifically demonstrate the promise of therapeutics targeting USP7 to improve the outcome of NB patients. NB patients with an intact USP7-HDM2-p53 axis may benefit from P22077 treatment either as single antitumor drug or as an effective adjunct to current chemotherapeutic regimens[2]. P22077 is a potent, selective, reversible inhibitor of USP7, a deubiquitinating enzyme that regulates the stability of p53-MDM2 pathway proteins [1][2] - Its mechanism of action involves binding to the active site of USP7, inhibiting its deubiquitinating activity, leading to stabilization of p53 and MDM2, upregulation of pro-apoptotic proteins (Bax, p21), downregulation of anti-apoptotic protein Bcl-2, and induction of cancer cell apoptosis [2] - P22077 exhibits significant in vitro and in vivo antitumor activity against neuroblastoma, supporting USP7 as a potential therapeutic target for neuroblastoma treatment [2] - The compound is used as a tool compound for studying USP7 function in p53 signaling and cancer biology, particularly in tumors with wild-type p53 [1][2] |
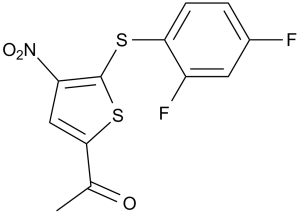
| 分子式 |
C12H7F2NO3S2
|
|
|---|---|---|
| 分子量 |
315.32
|
|
| 精确质量 |
314.984
|
|
| 元素分析 |
C, 45.71; H, 2.24; F, 12.05; N, 4.44; O, 15.22; S, 20.34
|
|
| CAS号 |
1247819-59-5
|
|
| 相关CAS号 |
|
|
| PubChem CID |
46931953
|
|
| 外观&性状 |
Light yellow to yellow solid powder
|
|
| LogP |
4.811
|
|
| tPSA |
116.43
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
20
|
|
| 分子复杂度/Complexity |
393
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
CC(C1=CC([N+]([O-])=O)=C(SC2=CC=C(F)C=C2F)S1)=O
|
|
| InChi Key |
RMAMGGNACJHXHO-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C12H7F2NO3S2/c1-6(16)11-5-9(15(17)18)12(20-11)19-10-3-2-7(13)4-8(10)14/h2-5H,1H3
|
|
| 化学名 |
1-(5-((2,4-difluorophenyl)thio)-4-nitrothiophen-2-yl)ethanone
|
|
| 别名 |
P22077; P-22077; 1247819-59-5; 1-[5-(2,4-Difluoro-phenylsulfanyl)-4-nitro-thiophen-2-yl]-ethanone; CHEMBL2159498; 1-(5-((2,4-Difluorophenyl)thio)-4-nitrothiophen-2-yl)ethanone; 1-(5-(2,4-difluorophenylthio)-4-nitrothiophen-2-yl)ethanone; 1-(5-((2,4-difluorophenyl)thio)-4-nitrothiophen-2-yl)ethan-1-one; P 22077.
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 50~63 mg/mL ( 158.57~199.79 mM)
Ethanol : ~1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.93 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 2 中的溶解度: 2% DMSO+30% PEG300+2% Tween80+66% ddH2O: 3mg/ml View More
配方 3 中的溶解度: 5 mg/mL (15.86 mM) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液; 超声助溶 (<60°C). 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1714 mL | 15.8569 mL | 31.7138 mL | |
| 5 mM | 0.6343 mL | 3.1714 mL | 6.3428 mL | |
| 10 mM | 0.3171 mL | 1.5857 mL | 3.1714 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|