| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
D2 Receptor ( Ki = 3.9 nM ); D3 Receptor ( Ki = 0.5 nM ); D4 Receptor ( Ki = 1.3 nM )
Dopamine D2 receptor (D2R) (Ki=2.2 nM) [1,2] Dopamine D3 receptor (D3R) (Ki=0.5 nM) [1,2] Dopamine D4 receptor (D4R) (Ki=5.8 nM) [2] |
|---|---|
| 体外研究 (In Vitro) |
普拉克索是一种用于治疗帕金森病症状的新型化学多巴胺激动剂,具有抗氧化活性,并且对缺氧缺血和甲基苯丙胺模型中的黑质多巴胺神经元具有神经保护作用。当与 SH-SY5Y 细胞孵育以及灌注到大鼠纹状体时,普拉克索均可降低甲基吡啶鎓离子 (MPP+) 产生的氧自由基水平。普拉克索还表现出对钙和磷酸盐或 MPP+ 诱导的线粒体过渡孔开放的浓度依赖性抑制。普拉克索剂量依赖性地降低多巴胺代谢物的水平,而纹状体多巴胺水平保持不变。普拉克索在这两种模型中都发挥作用,减少多巴胺周转的升高,以及减少 MAO 活性增加导致的羟自由基产生的升高,而 MAO 活性可能导致黑质纹状体神经元的氧化损伤。 Pramipexole (4-100 mM) 显着减弱 DA 或 L-DOPA 诱导的细胞毒性和细胞凋亡,这种作用不能被 D3 拮抗剂 U-99194 A 或 D2 拮抗剂雷氯必利阻断。 Pramipexole 还以剂量依赖性方式保护 MES 23.5 细胞免受过氧化氢诱导的细胞毒性。普拉克索可以有效抑制黑色素的形成,黑色素是DA或L-DOPA在无细胞系统中氧化产生的最终产物。
多巴胺受体激活作用:表达人D2R/D3R/D4R的CHO细胞经盐酸普拉克索一水合物(Pramipexole 2HCl Monohydrate)(0.1 nM-100 nM)处理后,药物作为完全激动剂,抑制毛喉素刺激的cAMP生成(D2R:EC50=3.1 nM;D3R:EC50=0.8 nM;D4R:EC50=7.2 nM),10 nM时诱导60%的细胞发生受体内化[1,2]。 - 神经保护活性:MPP⁺(500 μM)损伤的原代大鼠中脑多巴胺能神经元经盐酸普拉克索一水合物(Pramipexole 2HCl Monohydrate)(0.1 μM-10 μM)处理后,1 μM时细胞存活率提高52%(MTT法),活性氧(ROS)产生减少48%,凋亡率降低42%(流式细胞术)[1]。 - 多巴胺释放调节:大鼠纹状体突触体经盐酸普拉克索一水合物(Pramipexole 2HCl Monohydrate)(0.5 μM-20 μM)处理后,药物剂量依赖性抑制钾离子诱导的多巴胺释放,5 μM时实现50%抑制,机制为激活自身受体[2] |
| 体内研究 (In Vivo) |
在大脑中动脉闭塞(MCAO)诱导的大鼠缺血性中风模型中,普拉克索(1 mg/kg,静脉注射)在再灌注后24小时显著减少梗死体积(通过TTC染色评估)并改善神经功能评分。其保护作用与抑制缺血半暗带中线粒体细胞色素c释放和caspase-3激活相关[Dis Model Mech. 2019 Aug 1; 12(8): dmm033860.]
普拉克索(0.001-1 mg/kg sc)可降低小鼠的探索性运动活动。普拉克索(1 mg/kg,口服)能够显着降低增加的 DA 周转率,但仅降低 16%。 MPTP诱导小鼠帕金森病(PD)模型:20-25 g雄性C57BL/6小鼠腹腔注射MPTP(20 mg/kg),每日一次,连续5天。第6天起,口服灌胃盐酸普拉克索一水合物(Pramipexole 2HCl Monohydrate)(0.1 mg/kg、0.3 mg/kg、1 mg/kg),每日一次,连续14天。1 mg/kg剂量减少阿扑吗啡诱导的旋转行为68%,纹状体多巴胺水平升高2.3倍(HPLC检测)[1]。 - 大鼠自发活动模型:200-250 g雄性Wistar大鼠腹腔注射盐酸普拉克索一水合物(Pramipexole 2HCl Monohydrate)(0.5 mg/kg、1.5 mg/kg),1.5 mg/kg剂量在120分钟内增加45%的自发活动量,提高伏隔核多巴胺周转率[2] |
| 酶活实验 |
多巴胺受体的结合实验使用表达人D2、D3或D4受体的HEK293细胞膜制剂进行。将膜与[³H]螺哌隆(一种放射性标记配体)和递增浓度的普拉克索共同孵育,进行竞争结合实验。计算平衡解离常数(Ki),结果显示普拉克索对D3受体的亲和力最高,其次是D2和D4受体[Clin Ther, 2006. 28(8): p. 1065-78.]
多巴胺受体结合实验:从表达人D2R/D3R/D4R的CHO细胞或大鼠纹状体组织制备膜组分,将膜样品与[3H]-螺哌隆(0.5 nM)及不同浓度的盐酸普拉克索一水合物(Pramipexole 2HCl Monohydrate)(0.01 nM-100 nM)在25°C孵育60分钟。真空过滤分离结合态/游离态配体,液体闪烁计数器测量放射性,采用Cheng-Prusoff方程计算Ki值[1,2]。 - cAMP功能实验:将表达D2R/D3R/D4R的细胞接种于96孔板,用毛喉素(10 μM)预处理15分钟,再加入盐酸普拉克索一水合物(Pramipexole 2HCl Monohydrate)(0.1 nM-1 μM)孵育30分钟。ELISA法检测cAMP水平,确定受体介导抑制的EC50[1] |
| 细胞实验 |
散发性帕金森病与线粒体电子传递链复合物I的活性缺陷有关。这种电子传递链缺陷通过线粒体DNA传递,当在宿主细胞中表达时,会导致氧自由基产生增加,抗氧化酶活性增加,对程序性细胞死亡的易感性增加。普拉克索是一种用于治疗帕金森病症状的化学新型多巴胺激动剂,具有抗氧化活性,对缺氧缺血性和甲基苯丙胺模型中的黑质多巴胺神经元具有神经保护作用[1]。
多巴胺能神经元保护实验:分离原代大鼠中脑细胞培养7天,用盐酸普拉克索一水合物(Pramipexole 2HCl Monohydrate)(0.1 μM-10 μM)预处理1小时,再用MPP⁺(500 μM)处理24小时。MTT法评估细胞活力;荧光探针检测ROS;Annexin V/PI染色检测凋亡[1]。 - 多巴胺释放实验:匀浆离心法制备大鼠纹状体突触体,含氧Krebs-Ringer缓冲液重悬后,与盐酸普拉克索一水合物(Pramipexole 2HCl Monohydrate)(0.5 μM-20 μM)孵育30分钟,再用氯化钾(30 mM)刺激15分钟。收集上清液,高效液相色谱(HPLC)电化学检测多巴胺浓度[2] |
| 动物实验 |
0.001-1 mg/kg s.c.; 1 mg/kg, p.o.
Mice Pramipexole (SND 919; 2-amino-4,5,6,7-tetrahydro-6-propyl-amino-benzthiazole- dihydrochloride) was tested for its agonistic activity at pre- and postsynaptic dopamine (DA) receptors. L-Dihydroxyphenylalanine (L-dopa) accumulation in the rat striatum and limbic system and the alpha-methyltyrosine-induced reduction of DA were inhibited. Both effects were fully antagonized by haloperidol but not by the selective DA D1 receptor antagonist SCH 23390. Pramipexole decreased the levels of DA metabolites dose dependently, whereas striatal DA levels remained unchanged. In mice, pramipexole (0.001-1 mg/kg s.c.) reduced exploratory locomotor activity. In rats with unilateral striatal lesions, only weak ipsilateral rotation was produced by pramipexole at the highest dose. However, in rats with unilateral lesions of the medial forebrain bundle, pramipexole potently induced contralateral circling (ED50 0.026 mg/kg s.c.). In the N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) monkey model, pramipexole also had potent stimulatory effects. Finally, in haloperidol-sensitized monkeys, the substance did not elicit dyskinesia/dystonia when given alone, but rather inhibited those symptoms which had been induced by haloperidol (ED50 0.116 mg/kg i.m.). It is concluded that pramipexole has therapeutic potential for schizophrenic patients, as a result of its autoreceptor agonistic effects and its weak effects at normosensitive postsynaptic DA receptors. Furthermore, its potent stimulatory effects in DA-depleted animals suggest a possible use in the treatment of Parkinson's disease.[2] We found that pramipexole reduced the levels of oxygen radicals produced by methylpyridinium ion (MPP+) both when incubated with SH-SY5Y cells and when perfused into rat striatum. Pramipexole also exhibited a concentration-dependent inhibition of opening of the mitochondrial transition pore induced by calcium and phosphate or MPP+. These results suggest that pramipexole may be neuroprotective in Parkinson's disease by attenuating intracellular processes such as oxygen radical generation and the mitochondrial transition pore opening, which are associated with programmed cell death.[1] MPTP-induced PD model:Male C57BL/6 mice (20-25 g) were acclimated for 3 days before MPTP administration. Pramipexole 2HCl Monohydrate was dissolved in physiological saline and administered via oral gavage (0.1 mg/kg, 0.3 mg/kg, 1 mg/kg) daily for 14 days post-MPTP induction. Evaluate rotational behavior 30 minutes post-administration; euthanize mice to measure striatal dopamine levels via HPLC [1]. - Locomotor activity model:Male Wistar rats (200-250 g) were acclimated to activity chambers for 30 minutes. Pramipexole 2HCl Monohydrate was dissolved in physiological saline and administered via intraperitoneal injection (0.5 mg/kg, 1.5 mg/kg). Record total locomotor distance over 120 minutes; dissect nucleus accumbens to analyze dopamine turnover [2] |
| 药代性质 (ADME/PK) |
- pramipexole is rapidly absorbed after oral administration, with a time to peak plasma concentration (Tmax) of approximately 6 hours. Oral bioavailability is about 90%, and plasma protein binding is low (<20%). It has an elimination half-life of 8-12 hours and is primarily excreted unchanged in urine [1]
- pramipexole exhibits high blood-brain barrier permeability, with a brain-to-plasma concentration ratio of approximately 0.8. Its transport across the blood-brain barrier is mediated by the organic cation transporter 3 (OCT3) [2] Absorption:Oral bioavailability is 90% in humans; peak plasma concentration (Cmax) is reached at 2 hours post-oral administration (0.5 mg dose: Cmax=3.8 ng/mL) [2]. - Distribution:Volume of distribution (Vd) is 5.3 L/kg in humans; high blood-brain barrier penetration (brain/plasma concentration ratio=0.8-1.0) [2]. - Metabolism:Minimally metabolized in the liver (≤10% of dose), primarily excreted as unchanged drug [2]. - Excretion:80% of the dose is excreted in urine within 24 hours. Elimination half-life (t1/2) is 8-12 hours in humans [2]. - Plasma protein binding:Pramipexole 2HCl Monohydrate has a plasma protein binding rate of <15% in human plasma [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
- pramipexole showed low acute toxicity in animal studies, with a median lethal dose (LD50) >2000 mg/kg following oral administration in mice. Repeated dose studies in rats and dogs did not reveal significant hepatic or renal toxicity [1]
- pramipexole has minimal potential for drug-drug interactions due to its low plasma protein binding and limited metabolism by cytochrome P450 enzymes [1] - In mesencephalic cultures, pramipexole (1 μM) reduced levodopa-induced toxicity, as indicated by decreased ROS production, caspase-3 activation, and apoptotic cell death [4] Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of pramipexole during breastfeeding, but it suppresses serum prolactin and may interfere with breastfeeding. An alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information in nursing mothers was not found as of the revision date. Pramipexole lowers serum prolactin.[1] The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Acute toxicity:LD50 is 1160 mg/kg (oral) in rats, 980 mg/kg (oral) in mice [2]. - Chronic toxicity:Rats administered Pramipexole 2HCl Monohydrate (50 mg/kg/day, oral) for 6 months showed increased locomotor activity and mild weight gain (10%), no significant liver/kidney toxicity or hematological abnormalities [2]. - Clinical side effects:Sedation (20-25%), nausea (15-20%), dizziness (10-15%), and insomnia (8-10%) at therapeutic doses; no significant extrapyramidal symptoms [2] |
| 参考文献 | |
| 其他信息 |
- pramipexole is a non-ergoline dopamine agonist that selectively binds to D2, D3, and D4 receptors, with highest affinity for D3 receptors [1]
- The neuroprotective effects of pramipexole in various models (dopaminergic neurons, ischemic stroke) are linked to multiple mechanisms, including activation of BDNF/mTOR signaling (structural plasticity) and inhibition of mitochondrial dysfunction (reduced mPTP opening, cytochrome c release) [3,5] - pramipexole is clinically used for treating Parkinson's disease, leveraging its dopamine receptor agonist activity to modulate dopaminergic neurotransmission [1] Pramipexole hydrochloride is a hydrate that is the monohydrate of the dihydrochloride salt of pramiprexole. It has a role as a dopamine agonist and an antiparkinson drug. It contains a member of pramipexole hydrochloride anhydrous and a pramipexole(2+). A benzothiazole derivative and dopamine agonist with antioxidant properties that is used in the treatment of PARKINSON DISEASE and RESTLESS LEGS SYNDROME. See also: Pramipexole (annotation moved to); Pramipexole Dihydrochloride (annotation moved to). Drug Indication Pramipexole Accord is indicated in adults for treatment of the signs and symptoms of idiopathic Parkinson's disease, alone (without levodopa) or in combination with levodopa, i. e. over the course of the disease, through to late stages when the effect of levodopa wears off or becomes inconsistent and fluctuations of the therapeutic effect occur (end-of-dose or 'on-off' fluctuations). DAQUIRAN tablets are indicated for treatment of the signs and symptoms of advanced idiopathic Parkinson's disease in combination with levodopa, i. e. over the course of the disease, when the effect of levodopa wears off or becomes inconsistent and fluctuations of the therapeutic effect occur (end of dose or " on off" fluctuations). Combined vocal and multiple motor tic disorder (de la Tourette), Restless Legs Syndrome Combined vocal and multiple motor tic disorder (de la Tourette), Restless Legs Syndrome Pramipexole 2HCl Monohydrate is a non-ergot dopamine D2/D3/D4 receptor full agonist with neuroprotective activity [1,2]. - Mechanisms of action:Activates central dopamine autoreceptors and postsynaptic receptors to restore dopamine signaling in Parkinson's disease (PD); protects dopamineergic neurons from oxidative stress and apoptosis [1,2]. - Indications:Parkinson's disease (motor symptoms: tremor, rigidity, bradykinesia) and restless legs syndrome (RLS) [2]. - Administration:Oral dosing for adults (0.125-1.5 mg daily, divided into 3 doses or once-daily extended-release formulation) [2]. - Clinical advantage:Minimal liver metabolism, suitable for patients with hepatic impairment; low plasma protein binding, minimal drug-drug interaction risk [2]. - Caution:May cause somnolence; avoid driving or operating heavy machinery during treatment [2] |
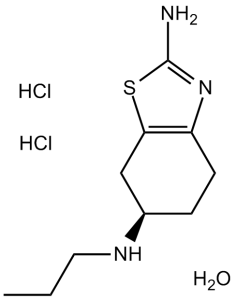
| 分子式 |
C10H21CL2N3OS
|
|
|---|---|---|
| 分子量 |
302.26
|
|
| 精确质量 |
301.078
|
|
| 元素分析 |
C, 39.74; H, 7.00; Cl, 23.46; N, 13.90; O, 5.29; S, 10.61
|
|
| CAS号 |
191217-81-9
|
|
| 相关CAS号 |
Pramipexole dihydrochloride; 104632-25-9; Dexpramipexole dihydrochloride; 104632-27-1; Pramipexole; 104632-26-0; Dexpramipexole; 104632-28-2
|
|
| PubChem CID |
166589
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 沸点 |
378ºC at 760 mmHg
|
|
| 熔点 |
290 °C(dec.)
|
|
| 闪点 |
182.4ºC
|
|
| 蒸汽压 |
9.93E-11mmHg at 25°C
|
|
| LogP |
4.094
|
|
| tPSA |
88.41
|
|
| 氢键供体(HBD)数目 |
5
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
17
|
|
| 分子复杂度/Complexity |
188
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
Cl[H].Cl[H].S1C(N([H])[H])=NC2=C1C([H])([H])[C@]([H])(C([H])([H])C2([H])[H])N([H])C([H])([H])C([H])([H])C([H])([H])[H].O([H])[H]
|
|
| InChi Key |
APVQOOKHDZVJEX-QTPLPEIMSA-N
|
|
| InChi Code |
InChI=1S/C10H17N3S.2ClH.H2O/c1-2-5-12-7-3-4-8-9(6-7)14-10(11)13-8;;;/h7,12H,2-6H2,1H3,(H2,11,13);2*1H;1H2/t7-;;;/m0.../s1
|
|
| 化学名 |
(6S)-6-N-propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine;hydrate;dihydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2 mg/mL (6.62 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2 mg/mL (6.62 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.0mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2 mg/mL (6.62 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 100 mg/mL (330.84 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3084 mL | 16.5420 mL | 33.0841 mL | |
| 5 mM | 0.6617 mL | 3.3084 mL | 6.6168 mL | |
| 10 mM | 0.3308 mL | 1.6542 mL | 3.3084 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
 |
|---|
 |
 |