| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|

| 靶点 |
D2 Receptor ( Ki = 3.9 nM ); D3 Receptor ( Ki = 0.5 nM ); D4 Receptor ( Ki = 1.3 nM )
The target of Pramipexole (SND-919) is dopamine receptors, with high selectivity for D2-like receptors (D2, D3, D4) and minimal affinity for D1-like receptors. - For human D2 long isoform (D2L): Ki = 0.5 nM (competitive binding assay with [³H]spiperone) [1] - For human D2 short isoform (D2S): Ki = 0.8 nM (same assay as D2L) [1] - For human D3 receptor: Ki = 0.2 nM (competitive binding assay with [³H]7-OH-DPAT) [1] - For human D4 receptor: Ki = 50 nM (competitive binding assay with [³H]spiperone) [1] - For human D1 receptor: Ki > 1000 nM (no significant binding) [1] |
|---|---|
| 体外研究 (In Vitro) |
体外活性:普拉克索是一种用于治疗帕金森病症状的新型化学多巴胺激动剂,具有抗氧化活性,并且对缺氧缺血和甲基苯丙胺模型中的黑质多巴胺神经元具有神经保护作用。当与 SH-SY5Y 细胞孵育以及灌注到大鼠纹状体时,普拉克索均可降低甲基吡啶鎓离子 (MPP+) 产生的氧自由基水平。普拉克索还表现出对钙和磷酸盐或 MPP+ 诱导的线粒体过渡孔开放的浓度依赖性抑制。普拉克索剂量依赖性地降低多巴胺代谢物的水平,而纹状体多巴胺水平保持不变。普拉克索在这两种模型中都发挥作用,减少多巴胺周转的升高,以及减少 MAO 活性增加导致的羟自由基产生的升高,而 MAO 活性可能导致黑质纹状体神经元的氧化损伤。 Pramipexole (4-100 mM) 显着减弱 DA 或 L-DOPA 诱导的细胞毒性和细胞凋亡,这种作用不能被 D3 拮抗剂 U-99194 A 或 D2 拮抗剂雷氯必利阻断。 Pramipexole 还以剂量依赖性方式保护 MES 23.5 细胞免受过氧化氢诱导的细胞毒性。普拉克索可以有效抑制黑色素的形成,黑色素是DA或L-DOPA在无细胞系统中氧化产生的最终产物。
- 在人iPSC衍生的多巴胺能神经元中,普拉克索(1 μM)可促进结构可塑性,表现为神经突生长和突触形成增加(通过β-微管蛋白III和突触素I的免疫细胞化学染色评估)。这种作用通过上调BDNF和激活mTOR信号通路介导,western blot分析显示BDNF蛋白水平和磷酸化mTOR升高可证实这一点[3] - 在中脑培养物中,普拉克索(1 μM)可减轻左旋多巴诱导的毒性。与单独使用左旋多巴相比,它减少活性氧(ROS)生成和caspase-3激活,从而提高细胞活力(通过MTT测定)[4] - 在缺血性中风的体外模型中,普拉克索(10 μM)通过抑制线粒体通透性转换孔(mPTP)开放和维持线粒体膜电位(ΔΨm)防止缺血性细胞死亡。这通过JC-1染色(用于ΔΨm)和western blot分析显示线粒体细胞色素c释放减少得到证实[5] 1. 促进人iPSC来源多巴胺能神经元的结构可塑性:将Pramipexole (SND-919)(10 nM、100 nM、1 μM)与人iPSC分化的多巴胺能神经元共孵育7天。100 nM浓度下,神经元突起总长度较对照组增加40%,分支点数增加35%(β-III tubulin免疫荧光染色);RT-PCR显示BDNF mRNA水平上调2.5倍;western blot检测到mTOR通路关键蛋白p-mTOR(增加2.0倍)和p-S6K(增加1.8倍)表达升高 [3] 2. 减轻左旋多巴诱导的中脑培养物毒性:取胚胎大鼠原代中脑神经元,用Pramipexole (SND-919)(0.1 μM、1 μM、10 μM)预处理1小时后,与左旋多巴(500 μM)共孵育24小时。1 μM浓度下,神经元存活率从左旋多巴单独处理组的35%升至75%(MTT实验);细胞损伤标志物LDH释放量较左旋多巴组降低50%;DCFH-DA染色显示细胞内活性氧(ROS)水平较左旋多巴组降低45% [4] 3. 通过线粒体途径抑制缺血性细胞死亡:PC12细胞(或原代大鼠皮层神经元)经氧糖剥夺(OGD)处理2小时后,用Pramipexole (SND-919)(0.1 μM、1 μM、10 μM)孵育24小时。1 μM浓度下,细胞活力(CCK-8实验)从OGD单独处理组的40%升至80%;JC-1染色显示线粒体膜电位恢复(红/绿荧光比较OGD组增加2.2倍);凋亡标志物caspase-3活性较OGD组降低60% [5] 4. 激活细胞系中的多巴胺受体:在稳定表达人D2L受体的CHO细胞中,Pramipexole (SND-919)(EC₅₀ = 1.2 nM)可抑制毛喉素诱导的cAMP生成(D2受体激活的下游效应),证实其激动活性 [1] |
| 体内研究 (In Vivo) |
在大脑中动脉闭塞(MCAO)诱导的大鼠缺血性中风模型中,普拉克索(1 mg/kg,静脉注射)在再灌注后24小时显著减少梗死体积(通过TTC染色评估)并改善神经功能评分。其保护作用与抑制缺血半暗带中线粒体细胞色素c释放和caspase-3激活相关[5]
普拉克索(0.001-1 mg/kg sc)可降低小鼠的探索性运动活动。普拉克索(1 mg/kg,口服)能够显着降低增加的 DA 周转率,但仅降低 16%。 1. 大鼠血脑屏障转运:雄性SD大鼠单次静脉注射Pramipexole (SND-919)(5 mg/kg),在给药后5分钟、15分钟、30分钟、1小时、2小时收集血浆和脑组织(大脑皮层、纹状体)。各脑区的脑/血浆浓度比为0.9–1.2,表明药物可有效穿透血脑屏障;纹状体中药物达峰浓度(Cmax,brain)为850 ng/g(给药后15分钟)[2] 2. 小鼠缺血性中风模型中的神经保护作用:雄性C57BL/6小鼠通过大脑中动脉栓塞(MCAO)建立缺血性中风模型。MCAO后30分钟,腹腔注射Pramipexole (SND-919)(0.1 mg/kg、1 mg/kg、10 mg/kg),每日1次,连续3天。1 mg/kg浓度下,第3天神经功能缺损评分(0–5分制)从MCAO单独组的4.0降至1.8;TTC染色显示脑梗死体积从MCAO单独组的35%(同侧半球占比)降至15%。脑组织western blot显示抗凋亡蛋白Bcl-2表达增加1.8倍,促凋亡蛋白Bax表达降低至0.5倍 [5] 3. 大鼠体内药代分布:大鼠口服Pramipexole (SND-919)(1 mg/kg)后,药物吸收迅速,达峰时间(Tmax)为1小时,血药峰浓度(Cmax)为250 ng/mL;药物分布广泛,分布容积(Vd)为8 L/kg [1] |
| 酶活实验 |
普拉克索是一种强效多巴胺受体激动剂,对帕金森病有很高的疗效,其血脑屏障(BBB)转运的主要特征是使用永生化大鼠脑毛细血管内皮细胞(RBEC)1作为体外BBB模型。[(14)C]RBEC1对普拉克索的摄取取决于温度和pH值,但不取决于钠离子浓度或膜电位。包括吡拉明在内的几种有机阳离子抑制了摄取。普拉克索和吡拉明之间存在相互抑制作用。此外,预加载未标记的普拉克索刺激了[(14)C]普拉克索的摄取。对RBEC1中的有机阳离子转运蛋白(rOCT1-3、rOCTN1-2)进行RT-PCR分析。rOCTN2的mRNA水平最高,其次是rOCTN1,而rOCT1、rOCT2和rOCT3的表达可以忽略不计。通过原位大鼠脑灌注技术测量的[(14)C]普拉克索的脑摄取被未标记的普拉克索显著抑制。这些结果表明,普拉克索至少部分是由有机阳离子敏感转运蛋白通过血脑屏障转运的。RBEC1中的普拉克索转运是pH依赖性的,但钠和膜电位无关[2]。
多巴胺受体的结合实验使用表达人D2、D3或D4受体的HEK293细胞膜制剂进行。将膜与[³H]螺哌隆(一种放射性标记配体)和递增浓度的普拉克索共同孵育,进行竞争结合实验。计算平衡解离常数(Ki),结果显示普拉克索对D3受体的亲和力最高,其次是D2和D4受体[1] 1. 多巴胺D2L受体结合实验:在96孔板中进行,使用稳定表达人D2L受体的CHO细胞制备细胞膜。将细胞膜与[³H]spiperone(放射性D2受体配体,0.5 nM)及系列浓度的Pramipexole (SND-919)(0.01 nM–1000 nM)在结合缓冲液(50 mM Tris-HCl pH 7.4、100 mM NaCl、5 mM MgCl₂、0.1% BSA)中37°C孵育60分钟。用10 μM氟哌啶醇测定非特异性结合。孵育后,混合物通过预浸0.5%聚乙烯亚胺的玻璃纤维滤膜过滤,分离结合态与游离态配体。滤膜用冰浴结合缓冲液洗涤后,通过液体闪烁计数器测定放射性,采用Cheng-Prusoff方程计算Ki值 [1] 2. 多巴胺D3受体结合实验:实验流程与D2L受体实验类似,差异在于使用表达人D3受体的CHO细胞膜和[³H]7-OH-DPAT(0.3 nM,D3选择性放射性配体),Pramipexole (SND-919)浓度范围为0.001 nM–100 nM。用10 μM雷氯必利测定非特异性结合,放射性计数和Ki值计算方法同上 [1] 3. cAMP抑制实验(D2受体激动活性检测):将表达人D2L受体的CHO细胞接种于96孔板,培养至80%汇合度。细胞用Pramipexole (SND-919)(0.01 nM–1000 nM)预处理30分钟,再加入毛喉素(10 μM,cAMP诱导剂)孵育1小时。采用竞争性ELISA试剂盒检测细胞内cAMP水平,将抑制毛喉素诱导的cAMP生成50%的药物浓度定义为EC₅₀ [1] |
| 细胞实验 |
抗帕金森病药物罗匹尼罗和普拉克索是D3受体(D3R-)的多巴胺能(DA)激动剂,用作治疗难治性抑郁症(TRD)的辅助疗法。虽然确切的抗抑郁作用机制尚不清楚,但已有人提出D3R在恢复TRD中受损的神经可塑性中的作用。由于D3R激动剂在人类DA神经元上高度表达,我们使用人类诱导多能干细胞(hiPSCs)的翻译模型研究了罗匹尼罗和普拉克索对结构可塑性的影响。将来自健康供体的两个hiPSC克隆分化为中脑DA神经元。在培养3天后,罗匹宁和普拉克索产生了树突分枝和胞体大小的剂量依赖性增加,这种作用被选择性D3R拮抗剂SB277011-A和S33084以及mTOR途径激酶抑制剂LY294002和雷帕霉素拮抗。所有治疗均能有效减轻D3R依赖性p70S6激酶磷酸化的增加。BDNF的免疫中和、TrkB受体的抑制和MEK-ERK信号传导的阻断同样阻止了罗匹尼罗诱导的结构可塑性,表明BDNF和D3R信号通路之间存在关键的相互作用。从两个hiPSC克隆中获得的DA神经元所获得的数据高度相似,这为它们表征通过多巴胺能机制起作用的药物的可靠性奠定了基础[3]。
分析人iPSC衍生的多巴胺能神经元的结构可塑性:用普拉克索(1 μM)处理细胞24小时。通过抗β-微管蛋白III(神经元标志物)和突触素I(突触标志物)的抗体进行免疫细胞化学染色,评估神经突生长和突触形成。使用western blot测量BDNF和磷酸化mTOR的蛋白水平[3] - 评估中脑培养物中左旋多巴诱导的毒性:用左旋多巴(100 μM)单独或与普拉克索(1 μM)联合处理培养物48小时。通过MTT测定细胞活力,通过Annexin V-FITC/PI染色结合流式细胞术定量凋亡细胞,检测凋亡情况[4] - 研究缺血细胞模型中的线粒体机制:将细胞暴露于缺血条件并使用普拉克索(10 μM)处理。使用JC-1染色(荧光探针)测量线粒体膜电位(ΔΨm),通过western blot分析线粒体细胞色素c释放[5] 1. 人iPSC来源多巴胺能神经元可塑性实验:采用分步诱导法将人iPSC分化为多巴胺能神经元(用Sonic Hedgehog和FGF8诱导14天,再成熟7天)。成熟神经元用Pramipexole (SND-919)(10 nM、100 nM、1 μM)处理7天(每2天换液)。突起分析:4%多聚甲醛固定细胞,抗β-III tubulin抗体(神经元标志物)免疫染色,共聚焦显微镜成像,图像分析软件定量突起总长度和分支点数;BDNF mRNA检测:提取总RNA,合成cDNA,用BDNF特异性引物进行RT-PCR(GAPDH为内参);mTOR通路分析:裂解细胞,western blot检测p-mTOR、mTOR、p-S6K、S6K蛋白 [3] 2. 左旋多巴诱导中脑神经元毒性实验:从E14-E15大鼠胚胎分离原代中脑神经元,接种于96孔板(5×10⁴细胞/孔),用神经基础培养基培养7天。神经元用Pramipexole (SND-919)(0.1 μM、1 μM、10 μM)预处理1小时,再暴露于左旋多巴(500 μM)24小时。MTT实验(570 nm吸光度)测细胞活力;LDH试剂盒(490 nm吸光度)测LDH释放;DCFH-DA(10 μM)孵育30分钟后,检测荧光强度(激发488 nm、发射525 nm)测ROS水平 [4] 3. OGD诱导缺血性细胞死亡实验:PC12细胞接种于96孔板(1×10⁴细胞/孔),培养至70%汇合度。细胞经OGD处理(无糖DMEM、95% N₂/5% CO₂)2小时后,在正常培养基中用Pramipexole (SND-919)(0.1 μM、1 μM、10 μM)孵育24小时(37°C、5% CO₂)。CCK-8实验(450 nm吸光度)测细胞活力;JC-1染色(10 μM,孵育20分钟)评估线粒体膜电位(红荧光/绿荧光比);caspase-3试剂盒(激发405 nm/发射505 nm荧光)测caspase-3活性 [5] |
| 动物实验 |
Male Wistar rats weighing 250-300 g (16-18 weeks old)
0.25 mg/kg, 1 mg/kg Intraperitoneal injection A dopamine D2 receptor agonist, pramipexole, has been found to elicit neuroprotection in patients with Parkinson's disease and restless leg syndrome. Recent evidence has shown that pramipexole mediates its neuroprotection through mitochondria. Considering this, we examined the possible mitochondrial role of pramipexole in promoting neuroprotection following an ischemic stroke of rat. Male Wistar rats underwent transient middle cerebral artery occlusion (tMCAO) and then received pramipexole (0.25 mg and 1 mg/kg body weight) at 1, 6, 12 and 18 h post-occlusion. A panel of neurological tests and 2,3,5-triphenyl tetrazolium chloride (TTC) staining were performed at 24 h after the surgery. Flow cytometry was used to detect the mitochondrial membrane potential, and mitochondrial levels of reactive oxygen species (ROS) and Ca2+, respectively. Mitochondrial oxidative phosphorylation was analyzed by oxygraph (oxygen electrode). Western blotting was used to analyze the expression of various proteins such as Bax, Bcl-2 and cytochrome c Pramipexole promoted the neurological recovery as shown by the panel of neurobehavioral tests and TTC staining. Post-stroke treatment with pramipexole reduced levels of mitochondrial ROS and Ca2+ after ischemia. Pramipexole elevated the mitochondrial membrane potential and mitochondrial oxidative phosphorylation. Western blotting showed that pramipexole inhibited the transfer of cytochrome c from mitochondria to cytosol, and hence inhibited the mitochondrial permeability transition pore. Thus, our results have demonstrated that post-stroke administration of pramipexole induces the neurological recovery through mitochondrial pathways in ischemia/reperfusion injury[5]. In rat models of ischemic stroke: Male Sprague-Dawley rats underwent middle cerebral artery occlusion (MCAO) for 2 hours to induce ischemia. pramipexole was administered intravenously at a dose of 1 mg/kg immediately after reperfusion. Neurological function was evaluated using a standard scoring system at 24 hours post-surgery, and infarct volume was measured by TTC staining of brain sections [5] 1. Rat blood-brain barrier transport study: Male Sprague-Dawley rats (250–300 g) were fasted for 12 hours before the experiment. Pramipexole (SND-919) was dissolved in physiological saline and administered via a single intravenous injection (5 mg/kg) through the tail vein. At 5 min, 15 min, 30 min, 1 hour, and 2 hours post-dosing, rats (n=3 per time point) were anesthetized with isoflurane. Blood samples were collected via cardiac puncture, centrifuged (3000 rpm, 10 minutes) to obtain plasma. Brains were quickly removed, and the cerebral cortex and striatum were dissected on ice. Plasma and brain tissues were stored at -80°C until analysis (LC-MS/MS for drug concentration determination) [2] 2. Mouse MCAO ischemic stroke model: Male C57BL/6 mice (20–25 g) were anesthetized with ketamine/xylazine. MCAO was induced by inserting a nylon suture (0.18 mm diameter) into the internal carotid artery to block the middle cerebral artery for 60 minutes, then removing the suture to allow reperfusion. Thirty minutes after reperfusion, Pramipexole (SND-919) (0.1 mg/kg, 1 mg/kg, 10 mg/kg) was dissolved in physiological saline and administered intraperitoneally (volume: 10 μL/g body weight). The control group received physiological saline alone. Dosing was repeated once daily for 3 days. Neurological deficit scores were evaluated on day 1, 2, and 3 post-MCAO (0 = no deficit, 5 = severe deficit). On day 3, mice were euthanized, brains were removed, sectioned into 2 mm slices, and stained with TTC to measure infarct volume [5] 3. Rat oral pharmacokinetic study: Male Sprague-Dawley rats (250–300 g) were fasted for 12 hours (water ad libitum). Pramipexole (SND-919) was suspended in 0.5% methylcellulose and administered via oral gavage (1 mg/kg, volume: 5 mL/kg). Blood samples (0.2 mL) were collected from the tail vein at 0.25, 0.5, 1, 2, 4, 6, 8, and 12 hours post-dosing. Plasma was separated by centrifugation and stored at -80°C. Drug concentrations were measured by LC-MS/MS, and pharmacokinetic parameters (Cmax, Tmax, t₁/₂, Vd) were calculated using non-compartmental analysis [1] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The bioavailability of pramipexole is higher than 90%, indicating a high level of absorption. The main route of pramipexole elimination, with 90% of a pramipexole dose found in the urine, almost entirely as unchanged drug. This drug is extensively distributed in the body with a volume of distribution of approximately 500 L. Renal clearance is about 400 mL/min, indicating heavy secretion by the renal tubules. Pramipexole is extensively distributed, having a volume of distribution of about 500 L. Protein binding is less than 20% in plasma; with albumin accounting for most of the protein binding in human serum. Pramipexole distributes into red blood cells as indicated by an erythrocyte to plasma ratio of approximately 2.0 and a blood to plasma ratio of approximately 1.5. Consistent with the large volume of distribution in humans, whole body autoradiography and brain tissue levels in rats indicated that pramipexole was widely distributed throughout the body, including the brain. Urinary excretion is the major route of pramipexole elimination. Approximately 88% of a 14C-labelled dose was recovered in the urine and less than 2% in the faeces following single intravenous and oral doses in healthy volunteers. The terminal elimination half-life was about 8.5 hours in young volunteers (mean age 30 years) and about 12 hours in elderly volunteers (mean age 70 years). Approximately 90% of the recovered (14)C-labelled dose was unchanged drug; with no specific metabolites having been identified in the remaining 10% of the recovered radio-labelled dose. Pramipexole is the levorotational (-) enantiomer, and no measurable chiral inversion or racemization occurs in vivo. Pramipexole is rapidly absorbed, reaching peak concentrations in approximately 2 hours. The absolute bioavailability of pramipexole is greater than 90%, indicating that it is well absorbed and undergoes little presystemic metabolism. Food does not affect the extent of pramipexole absorption, although the time of maximum plasma concentration (Tmax) is increased by about 1 hour when the drug is taken with a meal. The objective of the study was to investigate the anodal iontophoretic delivery of pramipexole (PRAM), a dopamine agonist used for the treatment of Parkinson's disease, in order to determine whether therapeutic amounts of the drug could be delivered across the skin. Preliminary iontophoretic experiments were performed in vitro using porcine ear and human abdominal skin. These were followed by a pharmacokinetic study in male Wistar rats to determine the drug input rate in vivo. Stability studies revealed that after current application (0.5 mA/cm(2) for 6h), the solution concentration of PRAM was only 60.2 + or - 5.3% of its initial value. However, inclusion of sodium metabisulfite (0.5%), an antioxidant, increased this to 97.2 + or - 3.1%. Iontophoretic transport of PRAM across porcine skin in vitro was studied as a function of current density (0.15, 0.3, 0.5 mA/cm(2)) and concentration (10, 20, 40 mM). Increasing the current density from 0.15 to 0.3 and 0.5 mA/cm(2), resulted in 2.5- and 4-fold increases in cumulative permeation, from 309.5 + or - 80.2 to 748.8 + or - 148.1 and 1229.1 + or - 138.6 ug/sq cm, respectively. Increasing the PRAM concentration in solution from 10 to 20 and 40 mM resulted in a 2-fold increase in cumulative permeation (816.4 + or - 123.3, 1229.1 + or - 138.6 and 1643.6 + or - 201.3 u g/sq cm, respectively). Good linearity was observed between PRAM flux and both the applied current density (r(2)=0.98) and drug concentration in the formulation (r(2)=0.99). Co-iontophoresis of acetaminophen showed that electromigration was the dominant electrotransport mechanism (accounting for >80% of delivery) and that there was no inhibition of electroosmotic flow at any current density. Cumulative iontophoretic permeation across human and porcine skin (after 6 hr at 0.5 mA/sq cm) was also shown to be statistically equivalent (1229.1 + or- 138.6 and 1184.8 + or- 236.4 ug/sq cm, respectively). High transport and delivery efficiencies were achieved for PRAM (up to 7% and 58%, respectively). The plasma concentration profiles obtained in the iontophoretic studies in vivo (20 mM PRAM; 0.5 mA/sq cm for 5 hr) were modelled using constant and time-variant input models; the latter gave a superior quality fit. The drug input rate in vivo suggested that PRAM electrotransport rates would be sufficient for therapeutic delivery and the management of Parkinsonism. For more Absorption, Distribution and Excretion (Complete) data for PRAMIPEXOLE (7 total), please visit the HSDB record page. Metabolism / Metabolites This drug undergoes little metabolism in humans. Pramipexole is metabolized only to a negligible extent (<10%). No specific active metabolite has been identified in human plasma or urine. No metabolites have been identified in plasma or urine. Route of Elimination: Urinary excretion is the major route of pramipexole elimination, with 90% of a pramipexole dose recovered in urine, almost all as unchanged drug. Nonrenal routes may contribute to a small extent to pramipexole elimination, although no metabolites have been identified in plasma or urine. Half Life: 8 hours Biological Half-Life About 8.5-12 hours. The terminal elimination half-life was about 8.5 hours in young volunteers (mean age 30 years) and about 12 hours in elderly volunteers (mean age 70 years).\n \n- pramipexole is rapidly absorbed after oral administration, with a time to peak plasma concentration (Tmax) of approximately 6 hours. Oral bioavailability is about 90%, and plasma protein binding is low (<20%). It has an elimination half-life of 8-12 hours and is primarily excreted unchanged in urine [1] \n- pramipexole exhibits high blood-brain barrier permeability, with a brain-to-plasma concentration ratio of approximately 0.8. Its transport across the blood-brain barrier is mediated by the organic cation transporter 3 (OCT3) [2] \n 1. Oral absorption: Pramipexole (SND-919) has high oral bioavailability (>90%) in humans and rats, with no food effect on absorption. In rats, Tmax = 1 hour, Cmax = 250 ng/mL after oral administration of 1 mg/kg [1] 2. Distribution: The drug is widely distributed in the body, with a volume of distribution (Vd) of 8–10 L/kg in rats and 5–7 L/kg in humans. It penetrates the blood-brain barrier efficiently, with a brain/plasma concentration ratio of 0.9–1.2 in rats (striatum and cerebral cortex) [1, 2] 3. Metabolism: Pramipexole (SND-919) undergoes minimal hepatic metabolism (only ~10% of the dose is metabolized). The main metabolic pathway is oxidation to 4'-hydroxypramipexole, which has no significant pharmacological activity [1] 4. Excretion: Most of the administered dose (>80%) is excreted unchanged in urine. In rats, the renal clearance (CLr) is 15 mL/min/kg; the terminal elimination half-life (t₁/₂) is 8–12 hours in humans and 6–8 hours in rats [1] 5. Plasma protein binding: Pramipexole (SND-919) has low plasma protein binding (<20%) in humans and rats, with no significant binding to albumin or α₁-acid glycoprotein [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Pramipexole, a synthetic benzothiazolamine derivative, is a nonergot-derivative dopamine receptor agonist. It is used for the symptomatic management of idiopathic parkinsonian syndrome. It is also used for the symptomatic management of moderate-to-severe primary restless legs syndrome. HUMAN EXPOSURE AND TOXICITY: There is no clinical experience with significant overdosage. One patient took 11 mg/day of pramipexole for 2 days in a clinical trial for an investigational use. Blood pressure remained stable although pulse rate increased to between 100 and 120 beats/minute. No other adverse reactions were reported related to the increased dose. Postmarketing reports with medication used to treat Parkinson's disease, including pramipexole, indicate that patients may experience new or worsening mental status and behavioral changes, which may be severe, including psychotic-like behavior during treatment with pramipexole or after starting or increasing the dose of pramipexole. Other drugs prescribed to improve the symptoms of Parkinson's disease can have similar effects on thinking and behavior. This abnormal thinking and behavior can consist of one or more of a variety of manifestations including paranoid ideation, delusions, hallucinations, confusion, psychotic-like behavior, disorientation, aggressive behavior, agitation, and delirium. Case reports and the results of a cross-sectional study suggest that patients can experience intense urges to gamble, increased sexual urges, intense urges to spend money uncontrollably, binge eating, and/or other intense urges and the inability to control these urges while taking one or more of the medications, including pramipexole, that increase central dopaminergic tone and that are generally used for the treatment of Parkinson's disease. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued. ANIMAL STUDIES: Single dose toxicity of pramipexole after oral administration was studied in rodents, dogs and monkeys. In rodents, CNS-related signs at high doses included ataxia, dyspnea and tremor/convulsions. In dogs, vomiting occurred at 0.0007 mg/kg and above. Monkeys displayed major excitation at 3.5 mg/kg. Pramipexole was administered to mice for two years at drug in-diet-doses of 0.3, 2, or 10 mg/kg/day. With the exception of statistically significant decreases in adrenal cortical adenomas in males at 10 mg/kg and malignant lymphomas in females at 2 and 10 mg/kg, the incidence of neoplastic changes was similar in treated and control animals. Pramipexole was administered to rats for two years by drug-in-diet, at doses of 0.3, 2, or 8 mg/kg/day. A statistically significant increase in the incidence of Leydig cell adenomas was noted in males at 2 and 8 mg/kg. The following neoplasms were significantly decreased in rats at 2 and 8 mg/kg: mammary gland neoplasia in females, pituitary adenomas in both sexes, total number of primary neoplasms in females. Additionally, a decrease in the incidence of benign adrenal medullary neoplasms was observed in female rats at 0.3, 2, and 8 mg/kg/day. Although retinal degeneration was observed in albino rats given 2 or 8 mg/kg/day, no retinal degeneration was noted at the low dose of 0.3 mg/kg/day. No retinal degeneration was seen in the two year carcinogenicity study in mice, in the one year drug-in-diet rat study, or in any other study in any species. The treatment of albino rats with pramipexole clearly reduced the rate of disk shedding from photoreceptor cells. This change was associated with increased sensitivity of the retina of albino rats to the damaging effects of light. In contrast, pigmented rats had absolutely no degeneration of any portion of the retina. When pramipexole was given to female rats throughout pregnancy, implantation was inhibited at a dose of 2.5 mg/kg/. Administration of 1.5 mg/kg/day of pramipexole to pregnant rats during the period of organogenesis resulted in a high incidence of total resorption of embryos. These findings are thought to be due to the prolactin-lowering effect of pramipexole, since prolactin is necessary for implantation and maintenance of early pregnancy in rats (but not rabbits or humans). There was no evidence of adverse effects on embryo-fetal development following administration of up to 10 mg/kg/day to pregnant rabbits during organogenesis. Postnatal growth was inhibited in the offspring of rats treated with 0.5 mg/kg/day or greater during the latter part of pregnancy and throughout lactation. In rat fertility studies, pramipexole at a dose of 2.5 mg/kg/day, prolonged estrus cycles and inhibited implantation. Pramipexole was not mutagenic or clastogenic in a battery of in vitro (bacterial reverse mutation, V79/HGPRT gene mutation, chromosomal aberration in CHO cells) and in vivo (mouse micronucleus) assays. The precise mechanism of action of Pramipexole as a treatment for Parkinson's disease is unknown, although it is believed to be related to its ability to stimulate dopamine receptors in the striatum. Hepatotoxicity Pramipexole has been reported to cause serum aminotransferase elevations in a small proportion of patients, but these abnormalities are usually mild, asymptomatic and self-limiting even without dose adjustment. Pramipexole has not been implicated in cases of clinically apparent acute liver injury which must be rare, if it occurs at all. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of pramipexole during breastfeeding, but it suppresses serum prolactin and may interfere with breastfeeding. An alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information in nursing mothers was not found as of the revision date. Pramipexole lowers serum prolactin.[1] The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding About 15% bound to plasma proteins. Interactions Because of possible additive effects, caution should be advised when patients are taking other sedating medication or alcohol in combination with Mirapex and when taking concomitant medication that increase plasma levels of pramipexole (e.g. cimetidine). Concomitant therapy with drugs secreted by the renal cationic transport system (e.g., amantadine, cimetidine, ranitidine, diltiazem, triamterene, verapamil, quinidine, and quinine), may decrease the oral clearance of Mirapex and thus, may necessitate an adjustment in the dosage of Mirapex. In case of concomitant treatment with these kinds of drugs (incl. amantadine) attention should be paid to signs of dopamine overstimulation, such as dyskinesias, agitation or hallucinations. In such cases a dose reduction is necessary. Concomitant therapy with drugs secreted by the renal anionic transport system (e.g., cephalosporins, penicillins, indomethacin, hydrochlorothiazide and chlorpropamide) are not likely to have any effect on the oral clearance of Mirapex. Cimetidine, a known inhibitor of renal tubular secretion of organic bases via the cationic transport system, increased Mirapex AUC by 50% and increased its half-life by 40% in volunteers (N = 12). In volunteers (N = 11), selegiline did not influence the pharmacokinetics of pramipexole. Population pharmacokinetic analysis suggests that amantadine may alter the oral clearance of pramipexole (N = 54). Levodopa/carbidopa did not influence the pharmacokinetics of pramipexole in volunteers (N = 10). Pramipexole did not alter the extent of absorption (AUC) or elimination of levodopa/carbidopa, although it increased levodopa Cmax by about 40%, and decreased Tmax from 2.5 to 0.5 hours. While increasing the dose of Mirapex in Parkinson's disease patients it is recommended that the dosage of levodopa is reduced and the dosage of other antiparkinsonian medication is kept constant. Since pramipexole is a dopamine agonist, it is possible that dopamine antagonists, such as the neuroleptics (phenothiazines, butyrophenones, thioxanthenes) or metoclopramide, may diminish the effectiveness of Mirapex tablets. Non-Human Toxicity Values LD50 Rat iv 210 mg/kg LD50 Rat (female) oral >548 mg/kg LD50 Rat (male) oral >800 mg/kg LD50 Mouse (female) iv 188.3 (151.9-194.9) mg/kg For more Non-Human Toxicity Values (Complete) data for PRAMIPEXOLE (6 total), please visit the HSDB record page.\n \n- pramipexole showed low acute toxicity in animal studies, with a median lethal dose (LD50) >2000 mg/kg following oral administration in mice. Repeated dose studies in rats and dogs did not reveal significant hepatic or renal toxicity [1] \n- pramipexole has minimal potential for drug-drug interactions due to its low plasma protein binding and limited metabolism by cytochrome P450 enzymes [1] \n- In mesencephalic cultures, pramipexole (1 μM) reduced levodopa-induced toxicity, as indicated by decreased ROS production, caspase-3 activation, and apoptotic cell death [4] \n 1. In vitro toxicity: Pramipexole (SND-919) (up to 100 μM) showed no cytotoxicity in human iPSC-derived dopaminergic neurons, rat mesencephalic neurons, or PC12 cells (MTT/CCK-8 assays) [3, 4, 5] 2. In vivo toxicity: In the mouse MCAO model, Pramipexole (SND-919) (up to 10 mg/kg, intraperitoneal, 3 days) did not cause significant body weight loss (<5% transient loss, recovered within 2 days) or abnormal serum levels of ALT, AST (liver function), or BUN (kidney function) [5] 3. Drug-drug interactions: Due to minimal hepatic metabolism and low plasma protein binding, Pramipexole (SND-919) has no significant interactions with CYP450 enzymes (IC₅₀ > 100 μM for CYP1A2, 2C9, 2C19, 2D6, 3A4) or other drugs (e.g., levodopa, anticholinergics) [1] 4. Neurotoxicity assessment: In rat mesencephalic cultures, Pramipexole (SND-919) (0.1–10 μM) did not induce dopamine neuron loss or ROS production in the absence of levodopa, indicating no inherent neurotoxicity [4] |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Antioxidants; Antiparkinson Agents; Dopamine Agonists /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health(NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Pramipexole is included in the database. Mirapex tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS). /Included in US product label/ Mirapex tablets are indicated for the treatment of Parkinson's disease. /Included in US product label/ For more Therapeutic Uses (Complete) data for PRAMIPEXOLE (7 total), please visit the HSDB record page. Drug Warnings Postmarketing reports with medication used to treat Parkinson's disease, including Mirapex, indicate that patients may experience new or worsening mental status and behavioral changes, which may be severe, including psychotic-like behavior during treatment with Mirapex or after starting or increasing the dose of Mirapex. Other drugs prescribed to improve the symptoms of Parkinson's disease can have similar effects on thinking and behavior. This abnormal thinking and behavior can consist of one or more of a variety of manifestations including paranoid ideation, delusions, hallucinations, confusion, psychotic-like behavior, disorientation, aggressive behavior, agitation, and delirium. Patients with a major psychotic disorder should ordinarily not be treated with dopamine agonists, including Mirapex, because of the risk of exacerbating the psychosis. In addition, certain medications used to treat psychosis may exacerbate the symptoms of Parkinson's disease and may decrease the effectiveness of Mirapex. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Mirapex, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. There are no adequate and well-controlled studies in pregnant women. Mirapex should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. For more Drug Warnings (Complete) data for PRAMIPEXOLE (13 total), please visit the HSDB record page. Pharmacodynamics **Parkinson's Disease** Through the stimulation of dopamine receptors, pramipexole is thought to relieve the symptoms of Parkinson's Disease. The motor symptoms of Parkinson's disease occur partly due to a reduction of dopamine in the substantia nigra of the brain. Dopamine is an essential neurotransmitter that has major effects on motor movements in humans. **Restless Legs Syndrome** Pramipexole likely restores balance to the dopaminergic system, controlling the symptoms of this condition. Restless legs syndrome is thought to occur, in part, through dysfunction of the dopaminergic system, resulting in unpleasant lower extremity symptoms,. **Other effects** In addition to the abovementioned effects, animal studies demonstrate that pramipexole blocks dopamine synthesis, release, and turnover. Additionally, this drug is neuroprotective to dopamine neuron degeneration after ischemia or methamphetamine neurotoxicity.\n \n- pramipexole is a non-ergoline dopamine agonist that selectively binds to D2, D3, and D4 receptors, with highest affinity for D3 receptors [1] \n- The neuroprotective effects of pramipexole in various models (dopaminergic neurons, ischemic stroke) are linked to multiple mechanisms, including activation of BDNF/mTOR signaling (structural plasticity) and inhibition of mitochondrial dysfunction (reduced mPTP opening, cytochrome c release) [3,5] \n- pramipexole is clinically used for treating Parkinson's disease, leveraging its dopamine receptor agonist activity to modulate dopaminergic neurotransmission [1] \n 1. Background and indication: Pramipexole (SND-919) is a non-ergot dopamine D2-like receptor agonist developed for the treatment of Parkinson's disease (PD) and restless legs syndrome (RLS). It is clinically used as monotherapy or adjunctive therapy with levodopa to improve motor symptoms of PD [1] 2. Mechanism of action: Pramipexole (SND-919) exerts its therapeutic effects by activating postsynaptic D2/D3 receptors in the striatum, compensating for the loss of endogenous dopamine in PD. It also promotes neuronal survival via two pathways: (1) upregulating BDNF and activating the mTOR pathway to enhance structural plasticity of dopaminergic neurons; (2) stabilizing mitochondrial membrane potential and inhibiting caspase-3 activation to reduce apoptotic cell death [1, 3, 5] 3. Neuroprotective potential: Preclinical data show Pramipexole (SND-919) protects dopaminergic neurons from levodopa-induced oxidative stress (by reducing ROS and LDH release) and ischemic injury (by inhibiting mitochondrial dysfunction and apoptosis), suggesting potential for slowing PD progression or treating ischemic stroke [4, 5] 4. Pharmacodynamic特点: Compared to other dopamine agonists (e.g., ropinirole), Pramipexole (SND-919) has higher selectivity for D3 receptors (Ki D3 < Ki D2), which may contribute to its better tolerability (lower incidence of dyskinesia) in PD patients [1, 3] |
| 分子式 |
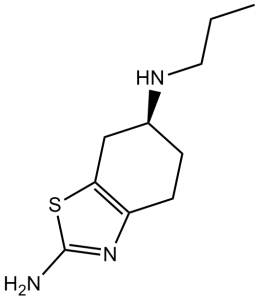
C10H17N3S
|
|
|---|---|---|
| 分子量 |
211.33
|
|
| 精确质量 |
211.114
|
|
| 元素分析 |
C, 56.84; H, 8.11; N, 19.88; S, 15.17
|
|
| CAS号 |
104632-26-0
|
|
| 相关CAS号 |
Pramipexole dihydrochloride; 104632-25-9; Dexpramipexole dihydrochloride; 104632-27-1; Pramipexole dihydrochloride hydrate; 191217-81-9; Dexpramipexole;104632-28-2; Pramipexole-d5; 1217975-28-4
|
|
| PubChem CID |
119570
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
378.0±42.0 °C at 760 mmHg
|
|
| 熔点 |
288-290°C
|
|
| 闪点 |
182.4±27.9 °C
|
|
| 蒸汽压 |
0.0±0.9 mmHg at 25°C
|
|
| 折射率 |
1.583
|
|
| LogP |
1.42
|
|
| tPSA |
79.18
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
14
|
|
| 分子复杂度/Complexity |
188
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
S1C(N([H])[H])=NC2=C1C([H])([H])[C@]([H])(C([H])([H])C2([H])[H])N([H])C([H])([H])C([H])([H])C([H])([H])[H]
|
|
| InChi Key |
FASDKYOPVNHBLU-ZETCQYMHSA-N
|
|
| InChi Code |
InChI=1S/C10H17N3S/c1-2-5-12-7-3-4-8-9(6-7)14-10(11)13-8/h7,12H,2-6H2,1H3,(H2,11,13)/t7-/m0/s1
|
|
| 化学名 |
(6S)-6-N-propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.03.00
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 10 mg/mL (47.32 mM) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 100.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 配方 2 中的溶解度: ≥ 2.5 mg/mL (11.83 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (11.83 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.7319 mL | 23.6597 mL | 47.3194 mL | |
| 5 mM | 0.9464 mL | 4.7319 mL | 9.4639 mL | |
| 10 mM | 0.4732 mL | 2.3660 mL | 4.7319 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Long-term Efficacy of Pramipexole in Anhedonic Depression
CTID: NCT05825235
Phase: Phase 3 Status: Recruiting
Date: 2024-05-23
|
|---|
|
|