| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|

| 靶点 |
PPARγ (Kd = 40 nM); PPARγ (EC50 = 60 nM); TRPC5 (EC50 = 30 μM); TRPM3
The targets of Rosiglitazone maleate and key parameters are as follows: - Peroxisome proliferator-activated receptor gamma (PPARγ): - High-affinity ligand for human PPARγ, with a dissociation constant (Ki) = 10 nM (measured by radioligand binding assay) [1] - Activates PPARγ-mediated transcriptional activity, with an half-maximal effective concentration (EC50) = 40 nM (using PPARγ-responsive luciferase reporter in CV-1 cells) [1] - For mouse PPARγ, EC50 for transcriptional activation = 15 nM (luciferase reporter assay in HeLa cells) [2] - Transient receptor potential melastatin 3 (TRPM3) channel: Inhibits TRPM3-mediated Ca²⁺ influx, with an half-maximal inhibitory concentration (IC50) = 1.2 μM (in HEK293 cells expressing human TRPM3) [4] - Transient receptor potential canonical 5 (TRPC5) channel: Enhances TRPC5-mediated cation current, with an EC50 = 0.8 μM (in HEK293 cells expressing human TRPC5) [4] - Neurotrophic factor-α1 (NTF-α1) promoter: Induces NTF-α1 transcription via PPARγ activation [3] . |
|---|---|
| 体外研究 (In Vitro) |
Rosiglitazonemaleate 对 PPARγ1 的 EC50 为 30 nM,对 PPARγ2 的 EC50 分别为 100 nM,对 PPARγ 的 Kd 约为 40 nM,使其成为一种强效选择性 PPARγ 激活剂。罗格列酮 (BRL49653, 0.1, 1, 10 μM) 有助于 C3H10T1/2 干细胞发育成脂肪细胞 [1]。化合物 6(罗格列酮)的 EC50 为 60 nM,可激活 PPARγ[2]。由于 PPARγ 与 NF-κ1 启动子结合,罗格列酮 (1 μM) 促进神经元中的基因转录。此外,罗格列酮 (1 μM) 以 NF-κ1 依赖性方式增加 BCL-2 表达,同时保护 Neuro2A 细胞和海马神经元免受氧化应激 [3]。 TRPM3 被罗格列酮完全阻断硝苯地平和 PregS 诱导的活性,IC50 值分别为 9.5 和 4.6 μM。然而,PPARγ不参与这一作用。大约 22.5 μM 的 IC50 表明罗格列酮在较大剂量下抑制 TRPM2。 EC50 约为 30 μM,使罗格列酮成为有效的 TRPC5 通道兴奋剂 [4]。
1. PPARγ激活及转录活性调控: - 在共转染人PPARγ表达质粒与PPARγ响应荧光素酶报告质粒的CV-1细胞中,Rosiglitazone maleate(1 nM-1 μM)处理24小时可浓度依赖性激活荧光素酶活性。100 nM时相对荧光素酶活性为溶媒对照的8.5倍,EC50=40 nM[1] - 在转染小鼠PPARγ与报告质粒的HeLa细胞中,Rosiglitazone maleate(0.1 nM-100 nM)诱导转录激活的EC50=15 nM。构效关系分析显示,其噻唑烷二酮环和对甲氧基苄基是PPARγ激动活性的关键结构[2] 2. 诱导NTF-α1转录的神经保护作用: - 在大鼠嗜铬细胞瘤PC12细胞中,Rosiglitazone maleate(0.1、1、10 μM)处理48小时: - NTF-α1 mRNA表达(RT-PCR检测)较对照分别升高1.8倍(0.1 μM)、2.5倍(1 μM)、3.2倍(10 μM)[3] - NTF-α1蛋白水平(Western blot检测)较对照分别升高1.6倍(1 μM)、2.3倍(10 μM)[3] - 对抗6-羟基多巴胺(6-OHDA,100 μM)诱导的细胞损伤:1 μM Rosiglitazone maleate可将凋亡率从38.7%±3.2%降至15.2%±2.1%(Annexin V-FITC/PI染色)[3] 3. TRPM3与TRPC5通道活性调控: - 在稳定表达人TRPM3的HEK293细胞中:Rosiglitazone maleate(0.1-10 μM)浓度依赖性抑制孕烯醇酮硫酸盐(PregS,TRPM3激动剂)诱导的Ca²⁺内流,IC50=1.2 μM;10 μM时抑制率>90%[4] - 在稳定表达人TRPC5的HEK293细胞中:Rosiglitazone maleate(0.1-5 μM)浓度依赖性增强卡巴胆碱诱导的TRPC5阳离子电流(全细胞膜片钳记录),EC50=0.8 μM;5 μM时电流振幅为对照的2.8倍[4] 。 |
| 体内研究 (In Vivo) |
在糖尿病大鼠中,罗格列酮(5 mg/kg,口服)可降低血糖水平。此外,罗格列酮降低了糖尿病组的 VCAM-1、TNF-α 和 IL-6 水平。当罗格列酮和氯沙坦联合使用时,与糖尿病组和洛沙坦治疗组相比,血糖水平升高。在从糖尿病大鼠分离的主动脉中,罗格列酮显着改善内皮功能障碍,明显降低对 PE 和 Ang II 的收缩反应以及增加 ACh 诱导的舒张 [5]。
1. 链脲佐菌素(STZ)诱导糖尿病大鼠中的疗效(联合洛沙坦): - 雄性SD大鼠通过单次腹腔注射STZ(60 mg/kg,溶于0.1 M柠檬酸盐缓冲液,pH 4.5)诱导糖尿病。72小时后选取空腹血糖(FBG)>16.7 mmol/L的大鼠,随机分为4组(每组6只): - 糖尿病对照组:溶媒(0.5%羧甲基纤维素,CMC)[5] - Rosiglitazone maleate组:3 mg/kg/天 Rosiglitazone maleate(溶于0.5% CMC)[5] - 洛沙坦组:10 mg/kg/天洛沙坦(溶于0.5% CMC)[5] - 联合组:3 mg/kg/天 Rosiglitazone maleate + 10 mg/kg/天洛沙坦[5] - 所有药物均通过口服灌胃给药,每日1次,连续8周。治疗结束时: - FBG:Rosiglitazone maleate组FBG从糖尿病对照组的28.5 mmol/L降至18.2 mmol/L;联合组进一步降至12.3 mmol/L[5] - 胰岛素抵抗:胰岛素抵抗指数(HOMA-IR)从糖尿病对照组的9.8降至Rosiglitazone maleate组的5.2、联合组的3.1[5] - 肾功能:尿白蛋白/肌酐比值(UACR)从糖尿病对照组的420 mg/g降至Rosiglitazone maleate组的250 mg/g、联合组的160 mg/g;血清肌酐从165 μmol/L降至Rosiglitazone maleate组的120 μmol/L、联合组的95 μmol/L[5] - 氧化应激:Rosiglitazone maleate组肾组织丙二醛(MDA)含量较糖尿病对照组降低35%,联合组降低52%;超氧化物歧化酶(SOD)活性Rosiglitazone maleate组升高1.4倍,联合组升高1.8倍[5] 。 |
| 酶活实验 |
脑过氧化物酶体增殖物激活受体γ(PPARγ)是配体依赖性转录因子核受体超家族的成员,参与神经保护。它被药物罗格列酮 激活,然后可以增加促生存蛋白B细胞淋巴瘤2(BCL-2),从而介导神经保护。然而,这种分子级联的机制仍然未知。在这里,我们发现神经保护蛋白神经营养因子-α1(NF-α1)也诱导BCL-2的表达,其启动子含有罗格列酮激活的PPARγ结合位点。罗格列酮治疗Neuro2a细胞和原代海马神经元可增加内源性NF-α1的表达,并防止H2 O2诱导的细胞毒性。随着NF-α1的增加,这些细胞中的BCL-2也增加了。当使用针对NF-α1的siRNA时,罗格列酮对BCL-2的诱导被阻止,罗格列列酮的神经保护作用降低。这些结果表明,罗格列酮激活的PPARγ直接诱导NF-α1的转录,有助于神经元的神经保护。我们提出了罗格列酮对氧化应激的神经保护的以下级联反应:罗格列酮进入神经元并与核内的过氧化物酶体增殖物激活受体γ(PPARγ)结合。PPARγ-罗格列酮复合物与神经营养因子-α1(NF-α1)启动子结合,激活NF-α1mRNA的转录,然后将其翻译成蛋白质。NF-α1是分泌的,与同源受体结合并激活细胞外信号调节激酶(ERK)通路。这反过来又增强了促生存蛋白B细胞淋巴瘤2(BCL-2)的表达和胱天蛋白酶3(Csp-3)的抑制,以介导氧化应激下的神经保护。Akt,蛋白激酶B(PKB)[3]。
1. PPARγ放射配体结合实验: - 将重组人PPARγ配体结合域(LBD)与[³H]标记的罗格列酮(0.5 nM)及不同浓度的未标记Rosiglitazone maleate(0.1 nM-1 μM)在结合缓冲液(20 mM Tris-HCl,pH 7.5,1 mM EDTA,10%甘油)中混合,4°C孵育16小时。 - 通过凝胶过滤柱分离游离放射配体与PPARγ-LBD-放射配体复合物,液体闪烁计数器检测复合物的放射性。 - 采用竞争结合方程计算解离常数(Ki),结果显示Ki=10 nM,证实Rosiglitazone maleate与PPARγ的高亲和力结合[1] 2. PPARγ转录活性实验(荧光素酶报告基因实验): - 将CV-1细胞接种于24孔板,用转染试剂共转染三种质粒:人PPARγ表达质粒(pCMV-PPARγ)、PPARγ响应报告质粒(pPPRE-luc,含3个PPAR响应元件)及海肾荧光素酶质粒(pRL-TK,内参)。 - 转染24小时后,更换为含Rosiglitazone maleate(1 nM-1 μM)或溶媒(DMSO,终浓度≤0.1%)的新鲜培养基,继续孵育24小时。 - 用被动裂解液裂解细胞,双荧光素酶报告基因检测系统测定荧光素酶活性,计算相对荧光素酶活性(萤火虫荧光素酶活性/海肾荧光素酶活性)以评估PPARγ激活水平[1] 3. TRPM3 Ca²⁺内流实验: - 稳定表达人TRPM3的HEK293细胞接种于96孔板,用5 μM Fluo-4 AM(Ca²⁺荧光探针)在汉克斯平衡盐溶液(HBSS)中37°C负载30分钟。 - 细胞用Rosiglitazone maleate(0.1-10 μM)或溶媒预处理5分钟,再加入10 μM孕烯醇酮硫酸盐(PregS,TRPM3激动剂)刺激。 - 酶标仪连续5分钟检测荧光强度(激发光488 nm,发射光525 nm),以峰值荧光强度量化Ca²⁺内流,拟合浓度-抑制曲线计算IC50[4] 。 |
| 细胞实验 |
本研究旨在为与葡萄糖稳态和代谢综合征相关的瞬时受体电位(TRP)通道的化学调节提供新的见解。人TRP美拉司他丁2(TRPM2)、TRPM3和TRP经典5(TRPC5)在人胚胎肾293细胞中有条件地过表达,并通过钙测量和膜片钳技术进行了研究。研究了罗格列酮和其他过氧化物酶体增殖物激活受体γ(PPAR-γ)激动剂。TRPM2在高达10μM的浓度下不受罗格列酮的影响,但在较高浓度下完全受到抑制(IC(50),约22.5μM)。TRPM3的抑制作用更强,其作用呈双相浓度依赖性,在低浓度(0.1-1μM)下抑制率约为20%,在高浓度(IC(50),5-10μM)时完全抑制。2-氯-5-硝基苯甲酰苯胺(GW9662)对PPAR-γ的拮抗作用并不能阻止罗格列酮对TRPM3的抑制作用。罗格列酮在≥10μM的浓度下强烈刺激TRPC5(EC(50),约30μM)。对TRPM3和TRPC5的影响迅速且可逆。罗格列酮和吡格列酮抑制TRPM3(IC(50),12μM),但对TRPC5没有影响,表明PPAR-γ或噻唑烷二酮部分与罗格列酮刺激TRPC5无关。罗格列酮相关但非噻唑烷二酮PPAR-γ激动剂N-(2-苯甲酰基苯基)-O-[2-(甲基-2-吡啶氨基)乙基]-l-酪氨酸(GW1929)是TRPM3和TRPC5的弱刺激剂。天然PPAR-γ激动剂15-脱氧前列腺素J(2)对TRPM3或TRPC5没有影响。数据表明,罗格列酮含有独立于PPAR-γ快速、强烈和差异调节TRP通道的化学部分,这可能导致该药物的生物学后果,并为新的TRP通道药理学提供了基础[4]。
1. PC12细胞神经保护实验: - PC12细胞以2×10⁵个/孔(6孔板,用于RT-PCR/Western blot)或5×10³个/孔(96孔板,用于凋亡检测)接种,用含10%胎牛血清(FBS)的RPMI 1640培养基在37°C、5% CO₂条件下培养[3] - NTF-α1表达检测:细胞用Rosiglitazone maleate(0.1、1、10 μM)处理48小时,TRIzol试剂提取总RNA,逆转录为cDNA后通过RT-PCR检测NTF-α1 mRNA(引物靶向NTF-α1与GAPDH);蛋白检测采用RIPA裂解液裂解细胞,抗NTF-α1抗体Western blot分析[3] - 凋亡检测:细胞用1 μM Rosiglitazone maleate预处理24小时,再暴露于100 μM 6-OHDA 24小时;Annexin V-FITC/PI染色后,流式细胞仪分析凋亡率[3] 2. HEK293细胞TRPC5电流记录实验: - 稳定表达人TRPC5的HEK293细胞用含10% FBS的DMEM培养,解离为单细胞后置于含细胞外液(140 mM NaCl、5 mM KCl、2 mM CaCl₂、1 mM MgCl₂、10 mM葡萄糖、10 mM HEPES,pH 7.4)的记录槽中[4] - 玻璃微电极(电阻2-4 MΩ)填充细胞内液(140 mM CsCl、5 mM EGTA、2 mM MgATP、10 mM HEPES,pH 7.2),形成全细胞膜片钳记录模式,膜电位钳制在-60 mV[4] - 细胞用Rosiglitazone maleate(0.1-5 μM)预处理3分钟,加入10 μM卡巴胆碱(TRPC5激动剂)激活电流,记录电流振幅并拟合浓度-响应曲线计算EC50[4] 。 |
| 动物实验 |
Rats are intravenously injected with 38 mg/kg streptozotocin and
after 48 h, diabetes is identified by urinary glucosuria and then random
blood sugar is measured and this day is regarded as day 0. Animals with
a serum glucose level of 220-300 mg/dL are selected to be used in this
study. Rats are randomly separated into five groups for daily drug
administration for 8 weeks: group 1: control nondiabetic rats given a
vehicle only (0.5 mL/kg of 0.5% carboxy methyl celleluse orally), group 2: control diabetic rats given a vehicle, group 3: diabetic rats receiving Rosiglitazone (5 mg/kg orally),
group 4: diabetic rats receiving losartan (2 mg/kg, orally), and group
5: diabetic rats receiving both Rosiglitazone and losartan
Rats 1. STZ-induced diabetic rat model (combination therapy with losartan): - Animals: Male SD rats (200-220 g, 8 weeks old) were acclimated for 1 week before the experiment. All rats were fed a standard diet and had free access to water [5] - Diabetes induction: Rats were fasted for 12 hours, then injected intraperitoneally with STZ (60 mg/kg, dissolved in 0.1 M citrate buffer, pH 4.5). The normal control group (not included in the 4 experimental groups) received an equal volume of citrate buffer. FBG was measured 72 hours after STZ injection; rats with FBG >16.7 mmol/L were considered diabetic and included in the study [5] - Grouping and administration: Diabetic rats were randomly divided into 4 groups (n=6 per group): - Diabetic control: Oral gavage of 0.5% CMC (0.2 mL/10 g body weight) once daily [5] - Rosiglitazone maleate: Oral gavage of 3 mg/kg/day Rosiglitazone maleate (dissolved in 0.5% CMC) once daily [5] - Losartan: Oral gavage of 10 mg/kg/day losartan (dissolved in 0.5% CMC) once daily [5] - Combination: Oral gavage of 3 mg/kg/day Rosiglitazone maleate + 10 mg/kg/day losartan (mixed in 0.5% CMC) once daily [5] - Treatment duration and sample collection: Drugs were administered for 8 weeks. Body weight was measured weekly; FBG was measured every 2 weeks via tail vein blood. At the end of treatment, rats were anesthetized with pentobarbital sodium (40 mg/kg, intraperitoneal injection). Blood was collected via abdominal aorta to detect serum insulin, creatinine, and oxidative stress markers (MDA, SOD). Urine was collected over 24 hours to measure UACR. Kidneys were excised: one part was fixed in 4% formalin for histopathological analysis, and the other part was homogenized to detect renal MDA and SOD [5] . |
| 毒性/毒理 (Toxicokinetics/TK) |
1. In vitro cytotoxicity:
- In PC12 cells and HEK293 cells, Rosiglitazone maleate at concentrations up to 20 μM had no significant effect on cell viability (MTT assay: viability >90% compared to the vehicle control), indicating low direct cytotoxicity [3,4] 2. In vivo toxicity in diabetic rats: - During the 8-week treatment with 3 mg/kg/day Rosiglitazone maleate: - Body weight: No significant difference in body weight change rate between the Rosiglitazone maleate group and the diabetic control group (weight gain: 5%-8% vs. 4%-6%) [5] - Hepatic and renal function: Serum alanine transaminase (ALT) and aspartate transaminase (AST) levels in the Rosiglitazone maleate group were within the normal range (ALT: 35-50 U/L, AST: 80-100 U/L), similar to the diabetic control group. No histopathological lesions (e.g., hepatocyte necrosis, renal tubular damage) were observed in the liver and kidney [5] - Other adverse effects: No signs of edema, cardiovascular dysfunction, or abnormal behavior were observed in the Rosiglitazone maleate group [5] ; |
| 参考文献 |
|
| 其他信息 |
Rosiglitazone Maleate is the maleate salt of rosiglitazone, an orally-active thiazolidinedione with antidiabetic properties and potential antineoplastic activity. Rosiglitazone activates peroxisome proliferator-activated receptor gamma (PPAR-gamma), a ligand-activated transcription factor, thereby inducing cell differentiation and inhibiting cell growth and angiogenesis. This agent also modulates the transcription of insulin-responsive genes, inhibits macrophage and monocyte activation, and stimulates adipocyte differentiation.
A thiazolidinedione that functions as a selective agonist for PPAR GAMMA. It improves INSULIN SENSITIVITY in adipose tissue, skeletal muscle, and the liver of patients with TYPE 2 DIABETES MELLITUS. See also: Rosiglitazone (has active moiety); Glimepiride; Rosiglitazone Maleate (component of); Metformin Hydrochloride; Rosiglitazone Maleate (component of). Drug Indication Rosiglitazone is indicated in the treatment of type 2 diabetes mellitus: as monotherapy-in patients (particularly overweight patients) inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intoleranceas dual oral therapy in combination with-metformin, in patients (particularly overweight patients) with insufficient glycaemic control despite maximal tolerated dose of monotherapy with metformin-a sulphonylurea, only in patients who show intolerance to metformin or for whom metformin is contraindicated, with insufficient glycaemic control despite monotherapy with a sulphonylureaas triple oral therapy in combination with-metformin and a sulphonylurea, in patients (particularly overweight patients) with insufficient glycaemic control despite dual oral therapy (see section 4. 4). Rosiglitazone is indicated as oral monotherapy in type 2 diabetes mellitus patients, particularly overweight patients, inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intolerance. Rosiglitazone is also indicated for oral combination treatment in type 2 diabetes mellitus patients with insufficient glycaemic control despite maximal tolerated dose of oral monotherapy with either metformin or a sulphonylurea: in combination with metformin particularly in overweight patients. in combination with a sulphonylurea only in patients who show intolerance to metformin or for whom metformin is contraindicated. Rosiglitazone is indicated as oral monotherapy in type 2 diabetes mellitus patients, particularly overweight patients, inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intolerance. Rosiglitazone is also indicated for oral combination treatment in type 2 diabetes mellitus patients with insufficient glycaemic control despite maximal tolerated dose of oral monotherapy with either metformin or a sulphonylurea: - in combination with metformin particularly in overweight patients. Â- in combination with a sulphonylurea only in patients who show intolerance to metformin or for whom metformin is contraindicated. Alzheimer's Disease 1. Background and mechanism of action: - Rosiglitazone maleate is a synthetic thiazolidinedione (TZD) class drug, and its core mechanism of action is as a selective agonist of PPARγ—a nuclear receptor that regulates glucose and lipid metabolism. By activating PPARγ, it promotes adipocyte differentiation, enhances insulin sensitivity in skeletal muscle and liver, and reduces insulin resistance [1,2] - For neuroprotection: Rosiglitazone maleate activates PPARγ to bind to the NTF-α1 promoter, inducing NTF-α1 transcription. NTF-α1 inhibits neuronal apoptosis and promotes neurite outgrowth, thereby exerting a neuroprotective effect against oxidative stress (e.g., 6-OHDA-induced injury) [3] - For ion channels: It exerts contrasting effects on TRPM3 (inhibition) and TRPC5 (activation) channels, which may be related to its non-PPARγ-mediated regulatory effects on cellular Ca²⁺ homeostasis [4] 2. Structure-activity relationship (SAR) of PPARγ agonism: - Literature [2] indicates that the thiazolidinedione ring of Rosiglitazone maleate is essential for PPARγ binding—removal of this ring completely abolishes agonistic activity. The p-methoxybenzyl group on the TZD ring enhances binding affinity to PPARγ, while substituting it with a methyl group reduces the EC50 for PPARγ activation by 5-fold [2] 3. Therapeutic potential: - Rosiglitazone maleate is primarily used for the treatment of type 2 diabetes mellitus (T2DM), especially in patients with severe insulin resistance. In combination with angiotensin II receptor blockers (e.g., losartan), it can further improve diabetic nephropathy (reducing UACR and protecting renal function) by synergistically inhibiting oxidative stress and inflammation [5] - It also shows potential in the treatment of neurodegenerative diseases (e.g., Parkinson's disease) due to its neuroprotective effect via NTF-α1 induction [3] ; |
| 分子式 |
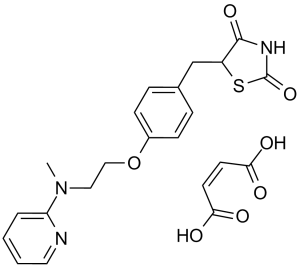
C18H19N3O3S.C4H4O4
|
|
|---|---|---|
| 分子量 |
473.5
|
|
| 精确质量 |
473.125
|
|
| 元素分析 |
C, 55.81; H, 4.90; N, 8.87; O, 23.65; S, 6.77
|
|
| CAS号 |
155141-29-0
|
|
| 相关CAS号 |
Rosiglitazone;122320-73-4;Rosiglitazone hydrochloride;302543-62-0
|
|
| PubChem CID |
5281055
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
585ºC at 760 mmHg
|
|
| 熔点 |
235-240°C
|
|
| 闪点 |
307.6ºC
|
|
| 折射率 |
1.688
|
|
| LogP |
2.38
|
|
| tPSA |
96.83
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
10
|
|
| 可旋转键数目(RBC) |
9
|
|
| 重原子数目 |
33
|
|
| 分子复杂度/Complexity |
588
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
CN(CCOC1=CC=C(C=C1)CC2C(=O)NC(=O)S2)C3=CC=CC=N3.C(=C\C(=O)O)\C(=O)O
|
|
| InChi Key |
SUFUKZSWUHZXAV-BTJKTKAUSA-N
|
|
| InChi Code |
InChI=1S/C18H19N3O3S.C4H4O4/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15;5-3(6)1-2-4(7)8/h2-9,15H,10-12H2,1H3,(H,20,22,23);1-2H,(H,5,6)(H,7,8)/b;2-1-
|
|
| 化学名 |
(Z)-but-2-enedioic acid;5-[[4-[2-[methyl(pyridin-2-yl)amino]ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.28 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.28 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.28 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1119 mL | 10.5597 mL | 21.1193 mL | |
| 5 mM | 0.4224 mL | 2.1119 mL | 4.2239 mL | |
| 10 mM | 0.2112 mL | 1.0560 mL | 2.1119 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01706211 | Completed | Drug: BRL 49653C Drug: Placebo |
Diabetes Mellitus Non Insulin Dependent Oral Agent Therapy |
National Taiwan University Hospital | October 1998 | Phase 3 |
| NCT00785213 | Completed Has Results | Drug: Rosiglitazone 4 mg Tablets Drug: Quinine Sulfate 324 mg Capsules |
Healthy | Mutual Pharmaceutical Company, Inc | September 2008 | Phase 1 |
| NCT01100619 | Completed | Drug: rosiglitazone Drug: XL184 |
Papillary Thyroid Cancer Follicular Thyroid Cancer |
Exelixis | April 2010 | Phase 1 |
| NCT00369174 | Completed | Drug: rosiglitazone maleate | Oral Leukoplakia | National Cancer Institute (NCI) | June 2006 | Phase 2 |
|
|---|
|
|
|
|---|
|
|