| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
HIF-PHD (hypoxia-inducible factor prolyl hydroxylases) (Ki = 5.3 nM)
|
|---|---|
| 体外研究 (In Vitro) |
缺氧诱导因子脯氨酰羟化酶(PHDs)抑制剂稳定缺氧诱导因子α,通过缺氧反应元件增加促红细胞生成素(EPO)的表达。因此,PHDs抑制剂已被开发为治疗贫血的新型药物。在这里,我们描述了TP0463518,2-[[1-[[6-(4-氯苯氧基)吡啶-3-基]甲基]-4-羟基-6-氧代-2,3-二氢吡啶-5-羰基]氨基]乙酸的体外和体内药理学特征,这是一种新型的强效PHDs抑制剂。TP0463518以5.3 nM的Ki值竞争性抑制人PHD2。TP0463518还抑制了人PHD1/3,IC50值为18和63 nM,以及猴子PHD2,IC50值是22 nM。
抑制PHDs活性[1] 如前所述,当HIF1α肽用作底物时,TP0463518对人和大鼠PHD2的IC50值分别为13和18 nM(Hamada等人,2018)。为了阐明TP0463518的抑制作用,我们使用HIF1α肽评估了人PHD1和PHD3的IC50值。TP0463518抑制PHD1,IC50值为18 nM(表1)。尽管TP0463518也抑制PHD3,但IC50值比PHD1/2高3.5倍和4.8倍。当使用HIF2α肽作为底物时,TP0463518以与使用HIF1α肽作为基质获得的效力相当的效力抑制了所有PHD。还研究了其他物种的抑制特征。TP0463518抑制猴子PHD2,IC50值为22nM。 为了阐明抑制模式,在每种TP0463518浓度下测量了最大活性和Km值。对于0、20和40 nM的TP0463518,最大活性(mP值)分别为46、47和47。在没有TP0463518的情况下,Km值为0.10μM,而在20和40 nM的TP0463558的情况下分别为0.32和0.61μM,表明存在竞争性抑制。使用双倒数图证实了竞争性抑制(图2)。基于竞争性抑制,TP0463518的Ki值计算为5.3 nM。 |
| 体内研究 (In Vivo) |
在正常小鼠和大鼠中,TP0463518分别在5和20mg/kg的剂量下显著提高了血清EPO水平。小鼠血清EPO和血清TP0463518水平的相关系数分别为0.95和0.92。TP0463518还以10mg/kg的剂量增加了5/6肾切除慢性肾病模型大鼠的血清EPO水平,血清EPO和血清TP0463558水平的相关系数为0.82。最后,研究了TP0463518对猴子的影响。TP0463518迅速被移除,半衰期为5.2小时,并在5 mg/kg的剂量下增加了EPO的曲线下面积(AUC)。EPO和TP0463528水平也存在相关性。这些结果表明,TP0463518诱导内源性EPO具有很强的药代动力学-药效学相关性,可能有助于肾性贫血患者理想的血红蛋白控制。1.
TP0463518对健康啮齿动物血清EPO水平的影响[1] 接下来,为了阐明TP0463518在啮齿动物中产生EPO的作用,对健康小鼠和大鼠口服单剂量TP0463528。Balb/c小鼠给药后6小时的血清EPO浓度如图3A所示。血清EPO浓度以剂量依赖的方式增加,在5mg/kg或更高的剂量下观察到显著的EPO产生作用。药代动力学-药效学(PK/PD)分析显示,血浆TP0463518浓度与血清EPO水平之间存在极好的相关性,相关系数为0.95(图3B)。 TP0463518对CKD模型大鼠血清EPO水平的影响[1] 为了研究TP0463518在CKD模型中产生EPO的作用,将TP0463528施用于5/6 Nx大鼠。与SD大鼠一样,在获得最大血清EPO浓度时评估血清EPO水平。在10mg/kg或更高的剂量下,血清EPO水平以剂量依赖的方式显著升高(图5A)。血清TP0463518浓度也与血清EPO浓度密切相关(图5B)。当血清TP0463518浓度相同时,5/6 Nx大鼠的血清EPO浓度与SD大鼠相当(图4B和图5B)。 TP0463518对猴子血清EPO水平的影响[1] 最后,在猴子(猕猴)身上研究了TP0463518的效果。血浆TP0463518浓度在施用20mg/kg TP0463528后1.6小时达到峰值,然后在分配阶段迅速下降(图6A)。消除阶段的T1/2为5.2小时。所有给药组的血清EPO浓度在给药后8小时达到峰值,然后在24小时下降(图6B)。当剂量达到或超过5mg/kg时,血清EPO AUC显著增加(图6C)。血清EPO AUC与血浆TP0463518 AUC相关(图6D)。 已知脯氨酰羟化酶(PHD)1/2/3泛抑制剂可能诱导肾脏和肝脏产生促红细胞生成素(EPO)。2-[1-[[6-(4-氯苯氧基)吡啶-3-基]甲基]-4-羟基-6-氧代-2,3-二氢吡啶-5-羰基]氨基]乙酸(TP0463518)是一种新型的PHD 1/2/3 pan抑制剂;然而,TP0463518给药后EPO产生的主要来源仍有待调查。我们通过测量正常和双侧肾切除大鼠的缺氧诱导因子2α(HIF-2α)、EPO mRNA和血清EPO水平,研究了TP0463518在诱导肾脏和肝脏产生EPO方面的作用。此外,我们还研究了肝脏来源的EPO是否改善了5/6肾切除(5/6Nx)大鼠的贫血。TP0463518几乎没有增加肾皮质中HIF-2α和EPO mRNA的表达水平,而口服40mg/kg的TP0463558显著增加了健康大鼠肝脏中HIF-2β水平,从0.27 fmol/mg增加到1.53fmol/mg,EPO mRNA表达水平增加了1300倍。以20mg/kg的剂量给药TP0463518后,整个肝脏中EPO mRNA的总表达水平是整个肾脏的22倍。在双侧肾切除的大鼠中,TP0463518在20 mg/kg的剂量下将血清EPO浓度从0提高到180 pg/ml。此外,重复服用10 mg/kg的TP0463528在第7天增加了5/6 Nx大鼠的网织红细胞计数,并在第14天提高了血红蛋白水平。本研究表明,TP0463518特异性诱导肝脏产生EPO并改善贫血。TP0463518的特征不仅可以更详细地了解红细胞生成中的PHD-HIF2α-EPO通路,而且可以为肾性贫血提供新的治疗选择。意义陈述:已知脯氨酰羟化酶(PHD)1/2/3泛抑制剂可能诱导肾脏和肝脏产生促红细胞生成素(EPO);然而,它们对肾脏EPO产生的影响已被证明因实验条件而异。作者发现,2-[[1-[[6-(4-氯苯氧基)吡啶-3-基]甲基]-4-羟基-6-氧代-2,3-二氢吡啶-5-羰基]氨基]乙酸(TP0463518),一种PHD 1/2/3 pan抑制剂,特异性诱导肝脏产生EPO,并且肝脏衍生的EPO在药理学上有效。对TP0463518作用的研究可能为开发肾性贫血患者的新治疗方案铺平道路[2]。 |
| 酶活实验 |
酶法测定[1]
使用荧光偏振进行PHDs抑制研究。将FITC-HIF和2-酮戊二酸与酶溶液在反应缓冲液(20mM Tris-HCl[pH 7.5],5mM KCl,1.5mM MgCl2,10μM硫酸亚铁,2mM抗坏血酸,1 mM DTT)与或不与不同浓度的TP0463518混合。FITC-HIF和2-酮戊二酸的浓度是每种酶Km值的两倍。反应温度为30°C,对每种PHD酶的反应时间进行优化,以获得初始速度(9-20分钟)。在反应结束时,将含有20mM EDTA和抗羟基化HIF抗体(Cell Signaling Technology,股份有限公司)的终止溶液加入到反应缓冲液中。然后,荧光(例如:480纳米,电磁:535nm)使用EnVision(PerkinElmer Japan Co.,有限公司)测量以计算毫极化(mP)值。mP值和相应的羟基化HIF浓度成正比,因此我们使用mP值作为活性。IC50值使用SAS 9.2版(日本东京SAS研究所)使用非线性最小二乘法计算。 为了确定抑制模式,用不同浓度的2-酮戊二酸(0.025-8μM)和TP0463518(0-40μM)测量了PHD2的活性。然后比较了每种TP0463518浓度对应的表观Vmax和Km。使用双倒数图确认了抑制模式。Ki值根据抑制模式计算(SAS 9.2)。 |
| 细胞实验 |
血清EPO的测定[1]
根据制造商手册,使用市售的EPO ELISA试剂盒测量小鼠、5/6 Nx大鼠和猴子的血清EPO水平,并稍作修改。使用夹心免疫测定系统测量健康大鼠的血清EPO水平。BioLegend和Meso Scale Diagnostics的大鼠EPO浓度被证实具有可比性。低于检测限的EPO水平被计算为零。 血浆/血清TP0463518浓度的测定[1] 使用液相色谱-串联质谱(LC-MS/MS)测量TP0463518的血浆/血清浓度,该质谱由LC-30AD HPLC系统和Triple Quad 5500质谱仪组成。 |
| 动物实验 |
Nine-week-old Balb/c mice were randomly assigned to a vehicle or a 5–40 mg/kg dose of TP0463518 group. The mice were orally treated with 0.5% methyl cellulose or a TP0463518 dosing suspension. Blood was collected at 6 h after administration from the orbital plexus under deep anesthesia, and euthanasia was performed without awakening. An aliquot of blood was mixed with EDTA, and the remaining blood sample was left to stand at room temperature for 15 min. The samples were then centrifuged (2130×g for 10 min at 4 °C) to prepare the plasma and serum.
For the healthy rats study, 7-week-old SD rats (Japan SLC, Inc.) were randomly assigned to a vehicle or 1.25–160 mg/kg dose of TP0463518 group. For the chronic kidney disease (CKD) model study, 5/6 nephrectomized SD (5/6 Nx) rats were prepared at Japan SLC, Inc., as follows. Two-thirds of the left kidney were resected at 4 weeks of age and the right kidney was removed at 5 weeks of age. The rats were then transferred to our facility and kept until 10 weeks of age, at which time they had developed anemia. The rats were assigned to a vehicle or a 2.5–80 mg/kg dose of TP0463518 group, while ensuring that there was no imbalance in the variance and mean of their whole-blood hemoglobin levels. SD rats and 5/6 Nx rats were orally treated with 0.5% methyl cellulose or a TP0463518 dosing suspension. Approximately 0.6 mL of blood was collected from the tail vein at 8 h (SD rats) or 4 h (5/6 Nx rats) after administration. The serum samples were prepared using the same method as that used for mice. Eight monkeys (9–12-year-old Macaca fascicularis.) were subjected to a fast for 16 h before administration and were re-fed at 8 h post-administration. Blood was collected from the cephalic vein or the femoral vein before (0) and 0.5, 1, 2, 4, 8, 12 and 24 h after administration. Plasma samples were prepared at all the time points, and serum samples were prepared at 0, 4, 8, 12 and 24 h after administration. The experiments were repeated weekly with increasing doses of TP0463518 from 0 (vehicle) to 20 mg/kg. |
| 药代性质 (ADME/PK) |
The half-life of TP0463518 (T1/2) in monkeys was 5.2 h. This value is very close to the predicted human T1/2 of 1.3–5.6 h estimated from pharmacokinetic parameters obtained in rats and dogs (Hamada et al., 2018). A T1/2 of 5 h might be a sufficient interval, since 2.5 mg/kg, which is one eighth of the effective dose of 20 mg/kg, was ineffective in the monkey study. Based on these results, clinical trials of TP0463518 are now being conducted as a once-daily preparation. Since PHDs inhibitors regulate a wide range of gene expressions, we believe that it is important to address concerns about mechanism-based side effects, especially VEGF induction. In the daprodustat, where the inhibitory activity is close to TP0463518 and the preparation is once-daily, a trend of VEGF was not clearly apparent (Holdstock et al., 2016; Akizawa et al., 2017). To conduct clinical trials in safe, we carefully titrated the dose and monitored VEGF in first-in-human study with healthy volunteers. [1]
|
| 参考文献 |
|
| 其他信息 |
PHD inhibitors protect HIFs alpha from proteasomal degradation by inhibiting HIF alpha hydroxylation (Schmid and Jelkmann, 2016). Subsequently, Epo, which is located downstream of the HIF response element, is upregulated and induces hematopoiesis (Haase, 2006, Percy et al., 2008). Recently, PHD inhibitors have been developed in clinical studies to ameliorate renal anemia, and a series of results showing a clinical proof-of-concept were reported from some companies (Akizawa et al., 2017, Martin et al., 2017, Provenzano et al., 2016). TP0463518 is a glycineamide-type PHDs inhibitor (Hamada et al., 2018) that is presently being examined in a clinical trial. In this report, we summarized the characteristics of TP0463518 in in vitro and in vivo studies. TP0463518 inhibited all human PHD1/2/3 on HIF1α, and it also inhibited rat and monkey PHD2. TP0463518 is a competitive inhibitor to 2-oxoglutarate, and its Ki value for human PHD2 was 5.3 nM. These findings suggest that the potency of TP0463518 is similar to daprodustat which is now in phase 3 trial (Ariazi et al., 2017). The IC50 value of TP0463518 for PHD3 was 3.5 and 4.8 times higher than those for PHD1/2, suggesting that TP0463518 is preferable to PHD1/2. Although TP0463518 had a preference for PHD1/2, the TP0463518 Cmax of monkey was much higher than the IC50 values (i.e., IC50 for human PHD3 63 nM was 27 ng/mL), so TP0463518 was considered to inhibit all the PHDs. TP0463518 also inhibited PHD2 when the substrate was HIF2α. As HIF2α plays an important role in EPO production (Appelhoff et al., 2004, Kapitsinou et al., 2010), the effects of TP0463518 on EPO production were then investigated in in vivo studies. [1]
TP0463518 showed a significant EPO-inducing effect in healthy mice and rats from doses of 5 and 20 mg/kg, respectively, with an excellent PK/PD correlation. In renal anemia, EPO production in response to hypoxia is impaired. To investigate the EPO-inducing effect of TP0463518 in renal anemic model animals, 5/6 Nx rats were dosed with TP0463518. TP0463518 induced EPO production with a strong PK/PD correlation. The serum EPO concentration in 5/6 Nx rats was comparable to that in healthy SD rats at the same exposure level. The number of renal EPO-producing (REP) cells in 5/6 Nx rats was estimated to be one sixth of those in healthy SD rats. Therefore, some mechanisms were assumed to increase the serum EPO levels to a certain level after TP0463518 administration regardless of the amount of remnant kidney. The following three possibilities could explain these mechanisms. [1] First, TP0463518 induced an approximately 6-times higher level of EPO production in the remnant damaged kidney in 5/6 Nx rats. When a unilateral ureteral obstruction was created in knockout mice lacking PHD1/2/3 in EPO-producing cells, the EPO mRNA levels in the damaged kidney were reportedly higher than those in the healthy kidney (Souma et al., 2016). In the paper, myofibroblasts, which had been REP cells before transformation, had the potency to express EPO in response to PHD deficiency. So, in our experiment, damaged REP cells in 5/6 Nx rats could have produced more EPO than normal REP cells. [1] A second possibility is that TP0463518 induced more EPO under hypoxic conditions in 5/6 Nx rats. One paper reported that hypoxia and ciclopirox (CPX), which is an iron chelator, synergistically increased reporter gene expressions via EPO HRE (Wanner et al., 2000). Iron chelators deprive iron from the enzyme and seems to inhibit the first step of the reaction (Hoffart et al., 2006). TP0463518 competed with 2-oxoglutarate and also seems to inhibit the first step of the reaction. Therefore, as in the case for CPX, a synergistic effect of TP0463518 and hypoxia might be expected in our experiments. [1] Finally, TP0463518 possibly increased extra-renal EPO production without enhancing EPO production in the kidney. Liver-specific PHD 1/2/3 triple knockout mice are known to have elevated liver EPO production (Minamishima and Kaelin, 2010). TP0463518 is a PHD1/2/3 pan-inhibitor, though the potency is slightly weak in PHD3. In this case, TP0463518 would not have reached REP cells since EPO production in the kidney is up-regulated by single suppression of PHD2 (Takeda et al., 2008). We are now investigating which organ is the main source of EPO and all these possibilities will be examined in future studies. [1] Next, the EPO-producing effect of TP0463518 was investigated in monkeys (Macaca fascicularis). The serum EPO AUC was correlated with the plasma TP0463518 AUC and increased significantly at a dose of 5 mg/kg or more. The EPO AUC, and not the EPO Cmax, is important for increasing blood hemoglobin levels (Masunaga et al., 1989). Because high levels of hemoglobin increase the risks of cardiovascular disease and stroke (Pfeffer et al., 2009, Singh et al., 2006), controlling the EPO AUC is very important for maintaining adequate levels of hemoglobin. Unlike exogenous erythropoiesis stimulating agent, as PHD inhibitor regulates endogenous EPO levels, a strong PK/PD correlation would lead to desirable hemoglobin control. A previous report suggested that a high dose of recombinant EPO used to treat anemia patients, which greatly exceeded normal physiologic ranges of EPO, might increase the risk of a cardiovascular event independent to blood pressure rise (Szczech et al., 2008, Inrig et al., 2012). In our experiment in monkeys, the serum EPO increased to 60 mU/mL at a dose of 20 mg/kg. This increase is comparable to the physiologic increase in endogenous EPO observed at high altitudes (Klausen et al., 1996) and is sufficient to ameliorate anemia when administered once daily in not only monkeys but also human (Akizawa et al., 2017, Flamme et al., 2014, Holdstock et al., 2016). As Flamme et al. discussed, erythropoiesis stimulating agents therapy, which leads serum EPO concentration over normal physiologic range, has a potential of long-term safety concern and a therapy with PHD inhibitors might not need such a high exposure of EPO. Therefore, TP0463518, which induced effective levels but not excess normal physiologic ranges of EPO, could ameliorate anemia with a lower risk of cardiovascular events that observed for recombinant EPO. [1] The systemic conditional knockout of PHD2 increases the serum VEGF concentration (Takeda et al., 2007). To reduce mechanism-based adverse effects, an interval during which the PHDs inhibitor does not work is considered important. The half-life of TP0463518 (T1/2) in monkeys was 5.2 h. This value is very close to the predicted human T1/2 of 1.3–5.6 h estimated from pharmacokinetic parameters obtained in rats and dogs (Hamada et al., 2018). A T1/2 of 5 h might be a sufficient interval, since 2.5 mg/kg, which is one eighth of the effective dose of 20 mg/kg, was ineffective in the monkey study. Based on these results, clinical trials of TP0463518 are now being conducted as a once-daily preparation. Since PHDs inhibitors regulate a wide range of gene expressions, we believe that it is important to address concerns about mechanism-based side effects, especially VEGF induction. In the daprodustat, where the inhibitory activity is close to TP0463518 and the preparation is once-daily, a trend of VEGF was not clearly apparent (Holdstock et al., 2016; Akizawa et al., 2017). To conduct clinical trials in safe, we carefully titrated the dose and monitored VEGF in first-in-human study with healthy volunteers. [1] Hypertension is a well-known adverse event observed in erythropoiesis therapy. Since TP0463518 induced EPO not exceeding the normal physiologic range, we believe that the risk of hypertension is low. Actually, there were no observed trends in blood pressure in phase 2 clinical study in vadadustat, which induces EPO not exceeding the normal physiologic range (Martin et al., 2017, Pergola et al., 2016), and hypertension was only observed in few patients in daprodustat, whose potency is close to TP0463518 (Akizawa et al., 2017). It will be soon reported that TP0463518 does not affect vital signs including blood pressure after single administration (Shinfuku et al., 2018). Based on these information, we believe that the risk of hypertension is low in TP0463518. Nevertheless, we plan to carefully monitor blood pressure in future clinical trials. [1] In summary, TP0463518 competitively inhibited human PHDs and also inhibited rat and monkey PHD2. TP0463518 increased serum EPO levels not only in healthy rodents, but also in anemic rats and monkeys. The serum EPO concentrations were well correlated with TP0463518 exposure in all the animals tested. TP0463518 is now being examined in a clinical trial with a once-daily dose regimen, and a clinical proof-of-concept for TP0463518 will be available in the future. TP0463518 is expected to become a new therapeutic option for the easy control of hemoglobin levels in renal anemia patients. |
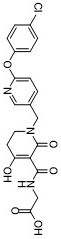
| 分子式 |
C20H18CLN3O6
|
|---|---|
| 分子量 |
431.826424121857
|
| 精确质量 |
431.088
|
| 元素分析 |
C, 55.63; H, 4.20; Cl, 8.21; N, 9.73; O, 22.23
|
| CAS号 |
1558021-37-6
|
| 相关CAS号 |
1558021-37-0
|
| PubChem CID |
73052863
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| LogP |
2.8
|
| tPSA |
129
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
692
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1C=CC(=CC=1)OC1=CC=C(C=N1)CN1C(C(C(NCC(=O)O)=O)=C(CC1)O)=O
|
| InChi Key |
HMMHKGLPKAQOOH-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C20H18ClN3O6/c21-13-2-4-14(5-3-13)30-16-6-1-12(9-22-16)11-24-8-7-15(25)18(20(24)29)19(28)23-10-17(26)27/h1-6,9,25H,7-8,10-11H2,(H,23,28)(H,26,27)
|
| 化学名 |
2-[[1-[[6-(4-chlorophenoxy)pyridin-3-yl]methyl]-4-hydroxy-6-oxo-2,3-dihydropyridine-5-carbonyl]amino]acetic acid
|
| 别名 |
TP0463518; 1558021-37-6; 2-(1-((6-(4-Chlorophenoxy)pyridin-3-yl)methyl)-4-hydroxy-2-oxo-1,2,5,6-tetrahydropyridine-3-carboxamido)acetic acid; V65GPE6NTB; 2-[[1-[[6-(4-chlorophenoxy)pyridin-3-yl]methyl]-4-hydroxy-6-oxo-2,3-dihydropyridine-5-carbonyl]amino]acetic acid; TP-0463518; (1-((6-(4-Chlorophenoxy)pyridin-3-yl)methyl)-2,4-dioxopiperidine-3-carbonyl)glycine; 2-((1-((6-(4-Chlorophenoxy)pyridin-3-yl)methyl)-4-hydroxy-6-oxo-2,3-dihydropyridine-5-carbonyl)amino)acetic acid;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 125 mg/mL (~289.47 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.82 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.82 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.82 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3157 mL | 11.5786 mL | 23.1573 mL | |
| 5 mM | 0.4631 mL | 2.3157 mL | 4.6315 mL | |
| 10 mM | 0.2316 mL | 1.1579 mL | 2.3157 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。