| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Notch (IC50 = 2.92±0.22 nM); APPL (IC50 = 2.64±0.30 nM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:YO-01027 直接与 γ-分泌酶复合物相互作用并靶向 N 端早老素片段。增加对 APPL 或 Notch 表达细胞施用的 YO-01027 浓度会导致 APPL CTF 片段逐渐积累,并以严格的剂量依赖性方式减少 NICD 的产生。 10 μM YO-01027 可减少乳腺癌干细胞 (BCSC) 的数量和活性。最近的一项研究表明,YO-01027 通过 Notch 抑制,在未分化细胞中在汇合前和汇合阶段以浓度依赖性方式损害粘蛋白蛋白 MUC16 生物合成,但在有丝分裂后分层细胞中则不然。激酶测定:对于YO-01027,使用0.1 nM至250 nM的不同药物浓度进行预实验,以确定YO-01027的有效线性范围和最大抑制剂量。在蛋白收获前 6 小时,诱导 Notch 或 APPL 表达后,将 YO-01027 以所需浓度添加至 S2 细胞培养基中。对于每个样品,裂解缓冲液中还包含相应浓度的 YO-01027,用于蛋白质提取和免疫印迹分析。细胞测定:将细胞以 ≤1 × 106 重悬于 100 μL 分选缓冲液(含 0.5% 牛血清白蛋白的 PBS、2 mM EDTA)中,并与预缀合一抗 BEREP4-FITC (1:10)、CD44-APC (1:10) 一起孵育。 20) 和 CD24-PE (1:10) 在 4 °C 下反应 10 分钟。将细胞用 PBS 清洗并以 800 × g 离心 2 分钟。为了进行分析,将细胞重悬于 500 μL 分选缓冲液中,使用 FACSCalibur 测量荧光并使用 WinMIDI 2.8 进行分析。为了进行分选,细胞与一抗孵育后重悬于 1× HBSS 中。使用 FACSAria 在 16 psi 下以 HBSS 作为鞘液对细胞进行分选。由 FACS 门控的 CD24low 细胞群是 CD24 阳性细胞加上所有 CD24 阴性细胞的最低五分位数。
本研究发现Notch3在未分化和已分化的HCLE和HCjE细胞中高表达,Notch1和Notch2的生物合成通过含血清培养基诱导分化而增强。在未分化细胞的融合前和融合阶段,用DBZ抑制Notch信号会以浓度依赖的方式破坏MUC16的生物合成,但在有丝分裂后的分层细胞中则不会。与蛋白水平相比,DBZ处理后MUC16转录物的数量没有显著减少,这表明Notch在转录后调控MUC16。DBZ处理的不同分化阶段上皮细胞的免疫印迹显示MUC1和MUC4的水平无差异。 结论:这些结果表明,MUC16的生物合成在上皮细胞分化的早期阶段受到Notch信号的转录后调控,并表明Notch激活有助于维持眼表粘膜表型。[3] |
| 体内研究 (In Vivo) |
YO-01027,在细胞注射当天和随后的每 3 天通过 ip 注射递送 1 mg/mL,YO-01027 显着减少 MCF7 但不减少 MDA-MB-231 肿瘤,并与对照小鼠相比增加潜伏期(18 -28 天)。 YO-01027 治疗的 MCF7 肿瘤确实形成,肿瘤体积显着减小。用 YO-01027 治疗 C57BL/6 小鼠,可抑制肠腺瘤中的上皮细胞增殖并诱导杯状细胞分化,且呈剂量依赖性。
|
| 酶活实验 |
为了确定 YO-01027 的有效线性范围和最大抑制剂量,利用 0.1 nM 至 250 nM 的不同药物浓度进行了初步研究。当诱导 Notch 或 APPL 表达时,在蛋白质收获前六小时,将 YO-01027 以适当的浓度添加到 S2 细胞培养基中。在用于蛋白质提取和免疫印迹分析的裂解缓冲液中,为每个样品额外添加适当浓度的 YO-01027。
|
| 细胞实验 |
将 ≤1 × 106 的重悬细胞与预缀合一抗 BEREP4-FITC (1:10)、CD44-APC (1:20) 和 CD24-PE (1:10) 一起孵育 10分钟,在 4 °C 下,在 100 μL 分选缓冲液(含 0.5% 牛血清白蛋白、2 mM EDTA 的 PBS)中。用 PBS 清洗后,将细胞以 800 × g 离心两分钟。将细胞重悬于500 μL分选缓冲液中进行分析,使用FACSCalibur测量荧光,使用WinMIDI 2.8进行分析。一抗孵育后,将细胞重悬于 1× HBSS 中进行分选。使用 FACSAria,以 HBSS 作为鞘液在 16 psi 下对细胞进行分选。 CD24 阳性细胞的最低五分位数加上所有 CD24 阴性细胞组成了 CD24low 细胞群,由 FACS 门控。
HCLE和HCjE细胞在不同的分化阶段生长,分别为未分化(预融合和融合)和分化(分层)上皮培养。Notch信号被γ-分泌酶抑制剂二苯氮平阻断(DBZ)。通过电泳和Western blot分析Notch细胞内结构域(Notch1至Notch3)和粘蛋白(MUC1, -4, -16)的存在。采用TaqMan实时聚合酶链反应检测粘蛋白基因表达[3]。 |
| 动物实验 |
Mice: In this study, male C57BL/6J wild-type (WT) and Apo E-/- mice are used. Four weeks of daily treatment are administered to Ang II-treated mice via intraperitoneal injection, starting the day before mini-pump implantation and continuing every day thereafter with either a saline vehicle or the γ-secretase inhibitor dibenzazepine (DBZ) (1 mg/kg/d, dissolved in saline). Using an automated tail-cuff system, blood pressure is measured in conscious mice. Every rodent is sedated. To facilitate additional histological and molecular analysis, the aortic tissues are removed.
|
| 参考文献 | |
| 其他信息 |
CLE and HCjE cells were grown at different stages of differentiation, representing nondifferentiated (preconfluent and confluent) and differentiated (stratified) epithelial cultures. Notch signaling was blocked with the γ-secretase inhibitor dibenzazepine (DBZ). The presence of Notch intracellular domains (Notch1 to Notch3) and mucin protein (MUC1, -4, -16) was evaluated by electrophoresis and Western blot analysis. Mucin gene expression was determined by TaqMan real-time polymerase chain reaction.
Results: Here we demonstrate that Notch3 is highly expressed in undifferentiated and differentiated HCLE and HCjE cells, and that Notch1 and Notch2 biosynthesis is enhanced by induction of differentiation with serum-containing media. Inhibition of Notch signaling with DBZ impaired MUC16 biosynthesis in a concentration-dependent manner in undifferentiated cells at both preconfluent and confluent stages, but not in postmitotic stratified cells. In contrast to protein levels, the amount of MUC16 transcripts were not significantly reduced after DBZ treatment, suggesting that Notch regulates MUC16 posttranscriptionally. Immunoblots of DBZ-treated epithelial cells grown at different stages of differentiation revealed no differences in the levels of MUC1 and MUC4.
Conclusions: These results indicate that MUC16 biosynthesis is posttranscriptionally regulated by Notch signaling at early stages of epithelial cell differentiation, and suggest that Notch activation contributes to maintaining a mucosal phenotype at the ocular surface.[3]
he gamma-secretase aspartyl protease is responsible for the cleavage of numerous type I integral membrane proteins, including amyloid precursor protein (APP) and Notch. APP cleavage contributes to the generation of toxic amyloid beta peptides in Alzheimer's disease, whereas cleavage of the Notch receptor is required for normal physiological signaling between differentiating cells. Mutagenesis studies as well as in vivo analyses of Notch and APP activity in the presence of pharmacological inhibitors indicate that these substrates can be differentially modulated by inhibition of mammalian gamma-secretase, although some biochemical studies instead show nearly identical dose-response inhibitor effects on Notch and APP cleavages. Here, we examine the dose-response effects of several inhibitors on Notch and APP in Drosophila melanogaster cells, which possess a homogeneous form of gamma-secretase. Four different inhibitors that target different domains of gamma-secretase exhibit similar dose-response effects for both substrates, including rank order of inhibitor potencies and effective concentration ranges. For two inhibitors, modest differences in inhibitor dose responses toward Notch and APP were detected, suggesting that inhibitors might be identified that possess some discrimination in their ability to target alternative gamma-secretase substrates. These findings also indicate that despite an overall conservation in inhibitor potencies toward different gamma-secretase substrates, quantitative differences might exist that could be relevant for the development of therapeutically valuable substrate-specific inhibitors.[1] Notch receptor signaling pathways play an important role not only in normal breast development but also in breast cancer development and progression. We assessed the role of Notch receptors in stem cell activity in breast cancer cell lines and nine primary human tumor samples. Stem cells were enriched by selection of anoikis-resistant cells or cells expressing the membrane phenotype ESA(+)/CD44(+)/CD24(low). Using these breast cancer stem cell populations, we compared the activation status of Notch receptors with the status in luminally differentiated cells, and we evaluated the consequences of pathway inhibition in vitro and in vivo. We found that Notch4 signaling activity was 8-fold higher in stem cell-enriched cell populations compared with differentiated cells, whereas Notch1 signaling activity was 4-fold lower in the stem cell-enriched cell populations. Pharmacologic or genetic inhibition of Notch1 or Notch4 reduced stem cell activity in vitro and reduced tumor formation in vivo, but Notch4 inhibition produced a more robust effect with a complete inhibition of tumor initiation observed. Our findings suggest that Notch4-targeted therapies will be more effective than targeting Notch1 in suppressing breast cancer recurrence, as it is initiated by breast cancer stem cells.[2] he self-renewing epithelium of the small intestine is ordered into stem/progenitor crypt compartments and differentiated villus compartments. Recent evidence indicates that the Wnt cascade is the dominant force in controlling cell fate along the crypt-villus axis. Here we show a rapid, massive conversion of proliferative crypt cells into post-mitotic goblet cells after conditional removal of the common Notch pathway transcription factor CSL/RBP-J. We obtained a similar phenotype by blocking the Notch cascade with a gamma-secretase inhibitor. The inhibitor also induced goblet cell differentiation in adenomas in mice carrying a mutation of the Apc tumour suppressor gene. Thus, maintenance of undifferentiated, proliferative cells in crypts and adenomas requires the concerted activation of the Notch and Wnt cascades. Our data indicate that gamma-secretase inhibitors, developed for Alzheimer's disease, might be of therapeutic benefit in colorectal neoplastic disease.[4] |
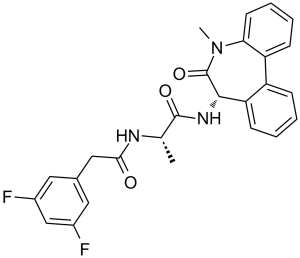
| 分子式 |
C26H23F2N3O3
|
|---|---|
| 分子量 |
463.48
|
| 精确质量 |
463.17
|
| 元素分析 |
C, 67.38; H, 5.00; F, 8.20; N, 9.07; O, 10.36
|
| CAS号 |
209984-56-5
|
| 相关CAS号 |
YO-01027;209984-56-5
|
| PubChem CID |
11454028
|
| 外观&性状 |
Yellow to orange solid powder.
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
801.3±65.0 °C at 760 mmHg
|
| 熔点 |
257-259ºC
|
| 闪点 |
438.4±34.3 °C
|
| 蒸汽压 |
0.0±2.8 mmHg at 25°C
|
| 折射率 |
1.637
|
| LogP |
4.6
|
| tPSA |
78.51
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
756
|
| 定义原子立体中心数目 |
2
|
| SMILES |
FC1C([H])=C(C([H])=C(C=1[H])C([H])([H])C(N([H])[C@@]([H])(C([H])([H])[H])C(N([H])[C@]1([H])C(N(C([H])([H])[H])C2=C([H])C([H])=C([H])C([H])=C2C2=C([H])C([H])=C([H])C([H])=C12)=O)=O)=O)F
|
| InChi Key |
QSHGISMANBKLQL-OWJWWREXSA-N
|
| InChi Code |
InChI=1S/C26H23F2N3O3/c1-15(29-23(32)13-16-11-17(27)14-18(28)12-16)25(33)30-24-21-9-4-3-7-19(21)20-8-5-6-10-22(20)31(2)26(24)34/h3-12,14-15,24H,13H2,1-2H3,(H,29,32)(H,30,33)/t15-,24-/m0/s1
|
| 化学名 |
(2S)-2-[[2-(3,5-difluorophenyl)acetyl]amino]-N-[(7S)-5-methyl-6-oxo-7H-benzo[d][1]benzazepin-7-yl]propanamide
|
| 别名 |
Dibenzazepine; YO01027; Iminostilbene; YO 01027; DBZ; 209984-56-5; (S)-2-(2-(3,5-Difluorophenyl)acetamido)-N-((S)-5-methyl-6-oxo-6,7-dihydro-5H-dibenzo[b,d]azepin-7-yl)propanamide; Dibenzazepine (Deshydroxy LY 411575); Deshydroxy LY-411575; DBZ; C26H23F2N3O3; YO01027; YO-01027; Deshydroxy LY-411575
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.39 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 2 中的溶解度: 0.5% hydroxyethyl cellulose: 6 mg/mL 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1576 mL | 10.7880 mL | 21.5759 mL | |
| 5 mM | 0.4315 mL | 2.1576 mL | 4.3152 mL | |
| 10 mM | 0.2158 mL | 1.0788 mL | 2.1576 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。